Yeah is a great way to make pasta, so simple and tasty. I just did it cowboy style with the chunks of potato and skin still on them. It worked out great! Old school italian. Need to figure out the sauce next. I like the canned organic crushed tomatoes. Would like to duplicate it since its good consistency and lightly cooked, but I usually just end up smashing tomatoes and cooking them straight up on pizza or chili/curry. Homemade tomato sauce would be amazing.
The hemp seed oil really makes the pesto. It has some active terpenes in it, very healty, get a health buzz off it since its a superfood.
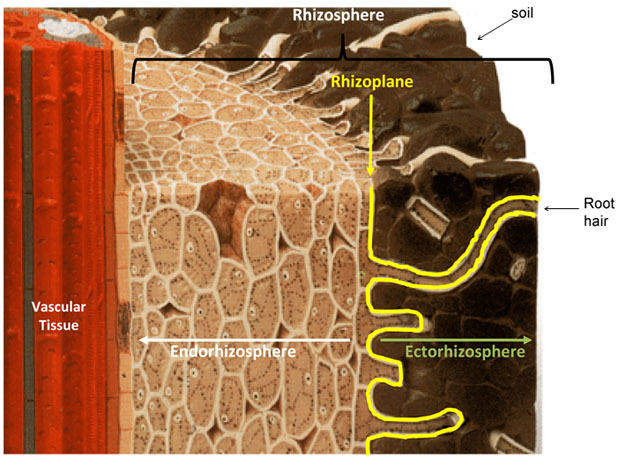
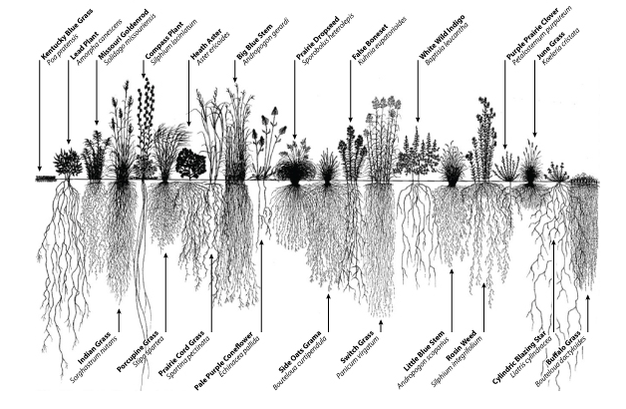
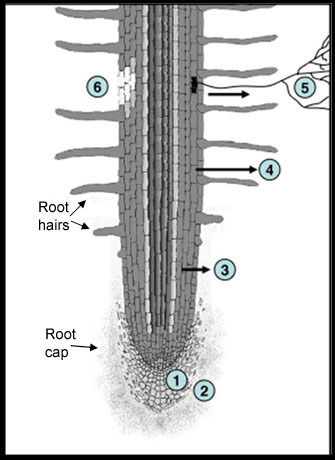
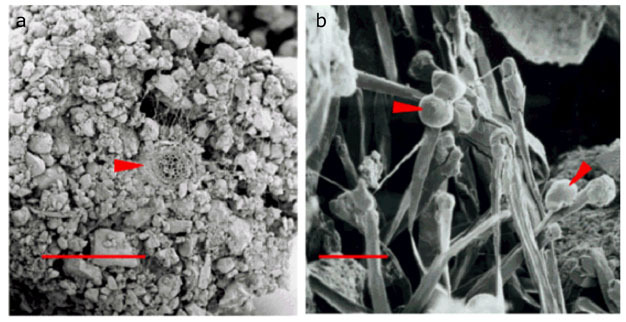
Going to check on the garden, had a quick rain a couple days ago and looking for males! Will be getting another good rain likely tuesday night! Love not having to water! God is giving me rest!
The hemp seed oil really makes the pesto. It has some active terpenes in it, very healty, get a health buzz off it since its a superfood.
Going to check on the garden, had a quick rain a couple days ago and looking for males! Will be getting another good rain likely tuesday night! Love not having to water! God is giving me rest!