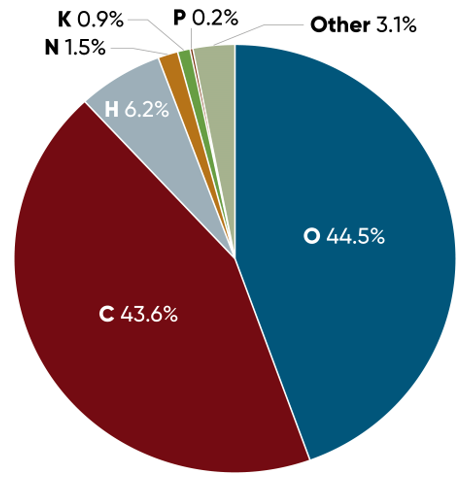
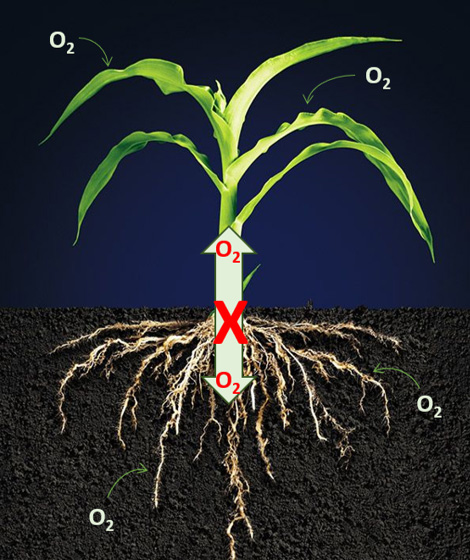
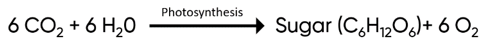
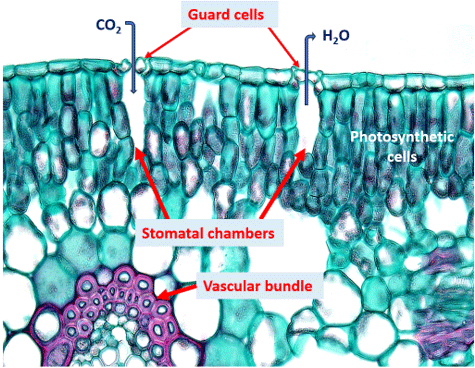
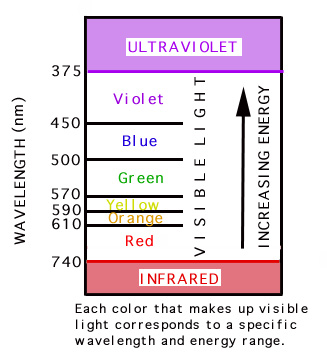
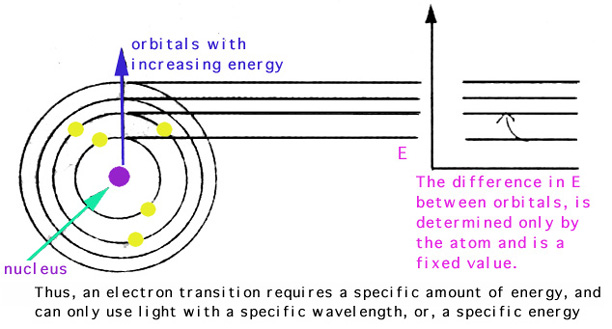
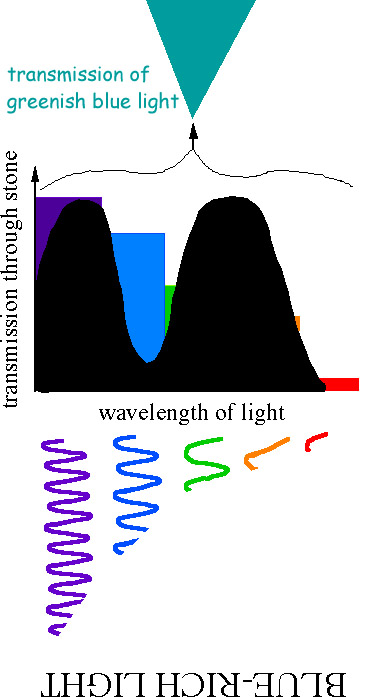
PH donner
Active member
Nice info!
I think you like tis too
It's in Dutch but Google translate is your friend
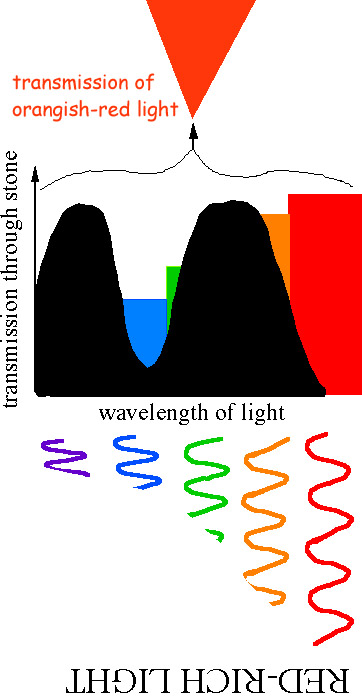
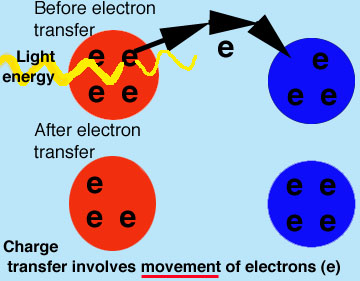
Stones give bread
I think you like tis too
It's in Dutch but Google translate is your friend
Stones give bread





.jpg)