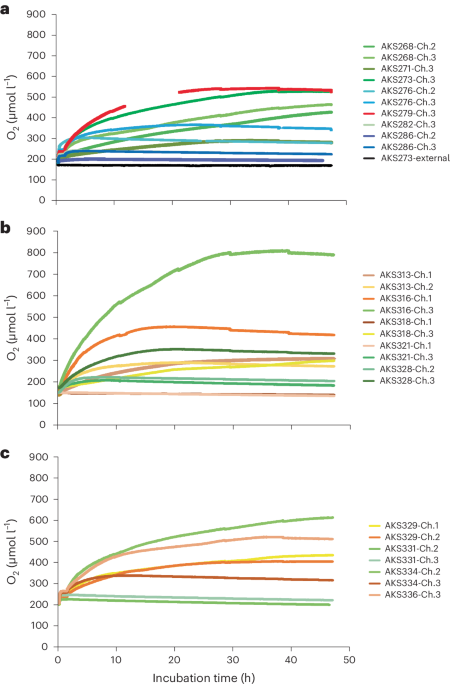
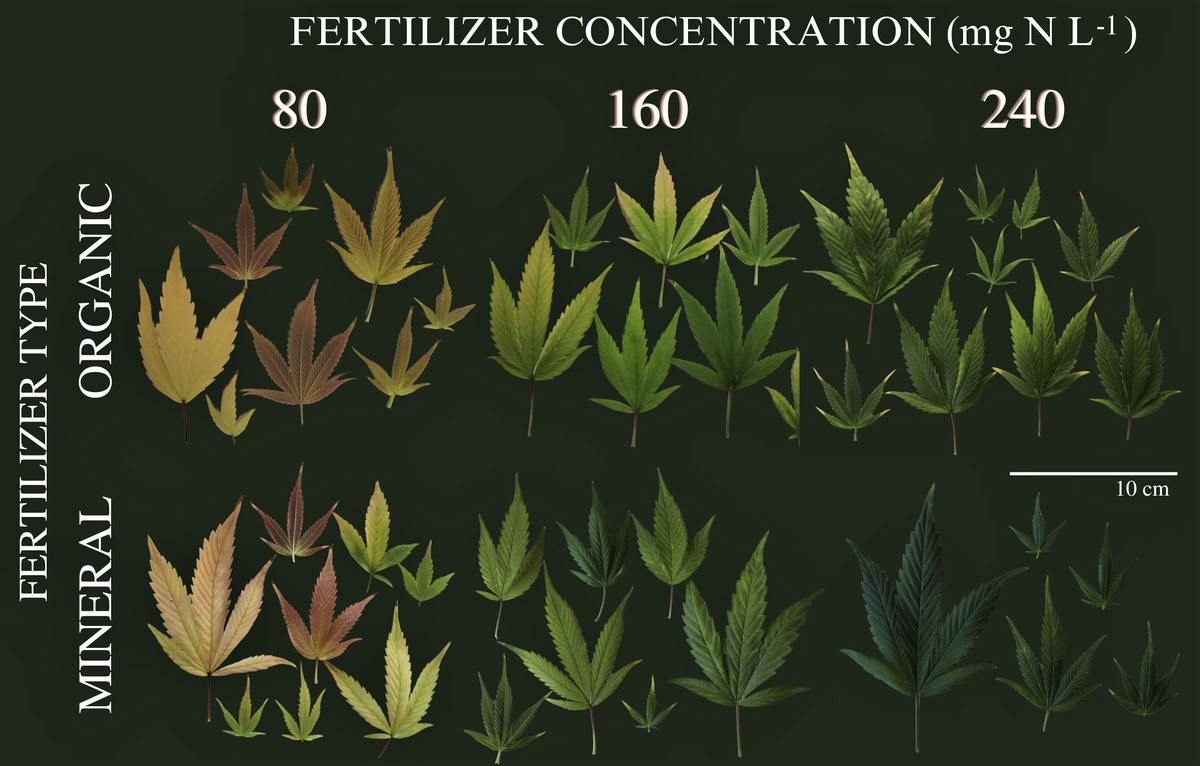
How do plant roots find the quickest way down? - Cornell Video
Most plants grow towards the light, but roots have to grow down into the soil to find water and nutrients. How do they do this? And what happens to a growing root when it encounters a rock? In this video a group of investigators from the Physics Department and from the Boyce Thompson Institute...