acespicoli
Well-known member
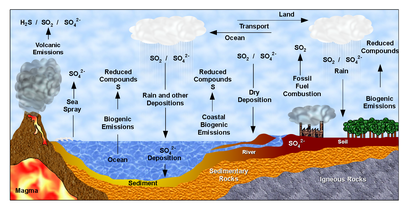
Plant Uptake of Mineral Nutrients from the Soil
How do plants overcome these tradeoffs in order to absorb nutrients from soil water into their root hairs? This process relies upon proton pumps, cation channels, and anion cotransporter channels present in the membranes of the root hairs as follows:- The epidermal tissue of root hairs is lined by proton pumps (H+ ATPases), which use ATP as an energy source to pump protons out of the cells and into the soils, against their electrochemical gradient. These proton pumps create a strong electrochemical gradient with a high concentration of protons and a strong positive charge outside of the cell, and a low concentration of protons and relatively negative charge inside of the cell. These protons pumped into the soil by the proton pumps cause two direct outcomes:
- The positively-charged protons bind to the negatively-charged clay particles in the soil, releasing the cations from the clay in a process called cation exchange. The cations then diffuse down their electrochemical gradient into the root hairs through cation channels. (The soil environment is highly positively charged, so it is energetically favorable for cations to move into the root hairs and out of the soil environment).
- The high concentration of protons in the soil creates a strong electrochemical gradient that favors transport of protons back into the root hairs. Plants use co-transport of protons down their concentration gradient as the energy source to also move anions against their concentration gradient into the root hairs. This process occurs through anion cotransporter channels. (The soil environment is highly positively charged, so it is energetically unfavorable for anions to leave the soil, but highly energetically favorable for protons to leave the soil).






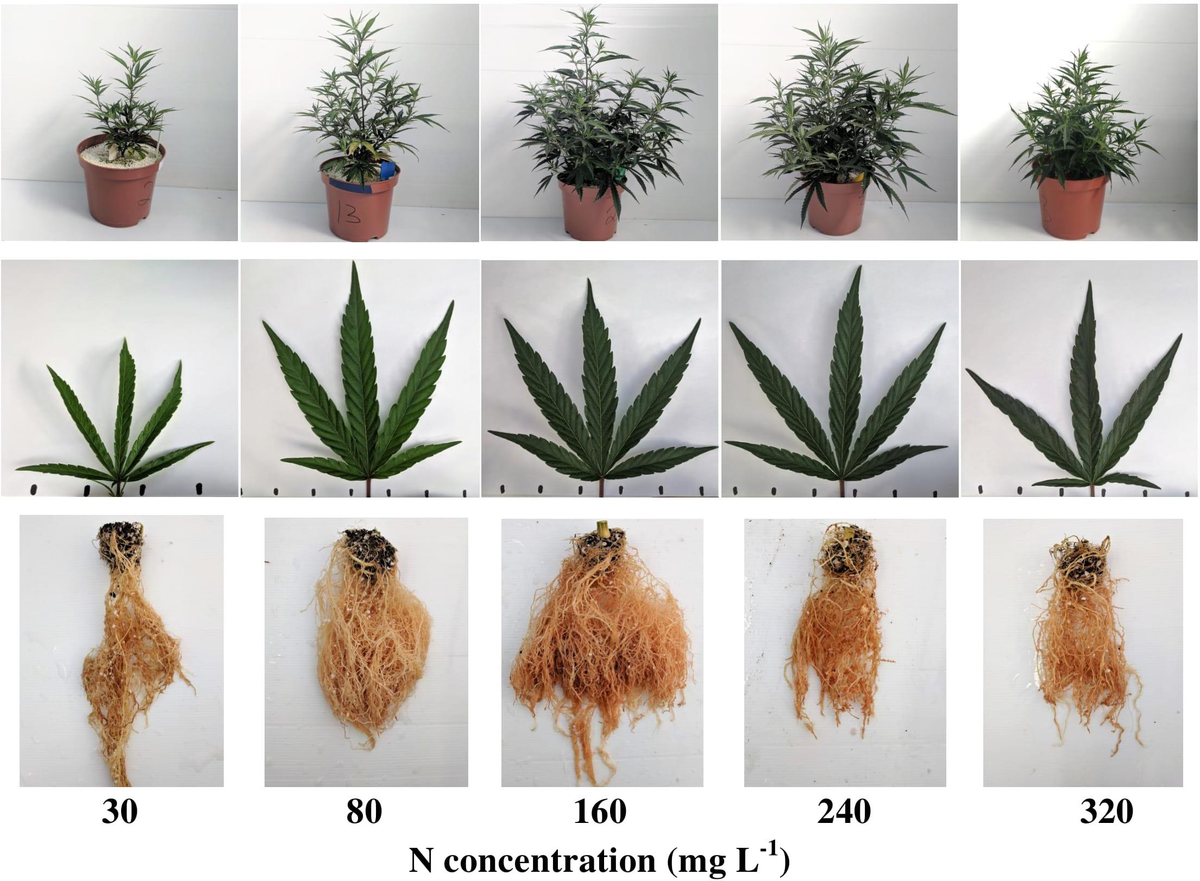











 best results with their seeds ive had
best results with their seeds ive had