I assume all these formulations with a calcium as high or nearly as high as the nitrogen are intended for growing in soil.
is this the gentleman that has been banned almost everywhere? If so, I have some questions on your bloom recirc formula
I assume all these formulations with a calcium as high or nearly as high as the nitrogen are intended for growing in soil.
"mj specific nutrient manufacturers"
I thought you would be the least person who use this expression.
Some of AN's products claimed to be cannabis specific (Kushie Kush) but officially they don't even mention the word cannabis on their other products.
Using shady terms like "our highly profitable plant" is not necessarily cannabis.
Other than AN I haven't seen a company who claims to sell nutes designed for cannabis. They try to suggest it but never claims it directly.
Anyway my point is:
AFAIK no real research has been done so far to determine the optimal nute levels for drug type cannabis plants so anyone who claims to sell nutes designed for cannabis lies.
GW Pharma might have done trials but it's not published.
If that is the case, what about Kushie Kush?
AN specifically state it's for cannabis.
What I don't understand is: why just 1 or 2 products are officially claimed to be for cannabis cultivation, why not the whole line?
I.) Notes:
...
5) After messing around with lots of mixes, I found the perfect mix of inexpensive salts. The mix provides N-P-K of 3-1-4 and K-Ca-Mg of 3.2-1.84-1 [i.e., 4-3.2-1.25, what you requested]; as well as providing sufficient Si, NO3:NH4 ratio of 14, etc. The following are the salts I used, including those you don't yet have. Using the following salts, excluding the micro's, increase the accuracy of the ppm calculations a great deal, which is a very good thing. Using all of the following salts I was able to reduce the price per unit, too:
...
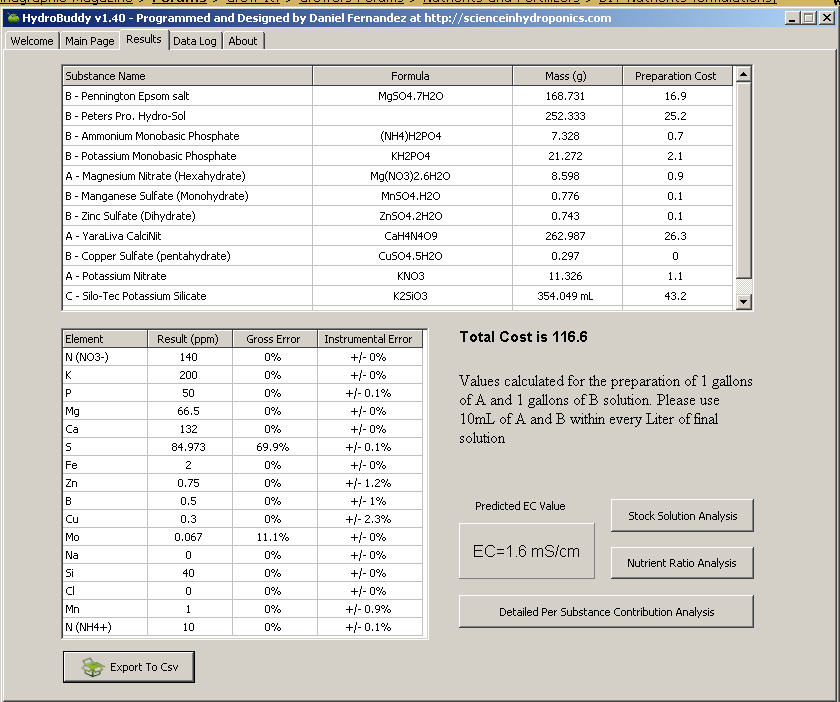
--> I attached two screen shots, the first one, "complete_mix_a", shows how to make the stock solutions (mixes) A, B and C. Ex., how much of each compound to add to each gallon to make stock solution (not shown is 0.361 grams of boric acid into mix B). That screen shot also shows the elemental profile by ppm, providing exactly what you asked for. The EC shown is 1.6 mS/cm, which is the EC of the fertagation water after the specified volumes of mix A, B and C are added. If we include the EC of your source water, i.e., 0.4 mS/cm, the total EC for your fertigtion tank will be 2.0 mS/cm; and I believe that is the EC you requested, correct?
II.) Mixing notes for stock solution:
a) when weighing dry salts use two scales: one that measures down to 0.001 gram and is accurate to 0.001 gram (jewelry scale for micro-nutrients), and one that measures to 1 kilogram and accurate to 0.01 gram, better yet 0.001 gram. Following is an example of en Ebay search for 0.001 gram scales: http://shop.ebay.com/items/?_nkw=sc...pt=1&_sadis=&LH_CAds=&clk_rvr_id=248929254212
b) when measuring liquid volume, ex., for Silo-Tec potassium silicate, use a good graduated cylinder, and a syringe for single mL and less than mL measurements. However, we can find the specific gravity of Silo-Tec and then use weight, instead of volume, to measure out Silo-Tec for mix C.
c) fill jug for mix A with 1/2 gallon distilled or deionized water, then add the salt compounds for mix A, shake and dissolve with each addition. Then add the other 1/2 gallon distilled or deionized water and shake.
d) do the same as above (re: mix A), when mixing B, and C.
III.) Mixing notes for fertigation tank (working solution):
a) fill tank to 1/2 volume with source water needed, ex., if you need 100 liter, fill with 50 liter.
b) add mix C (potassium silicate) at 10 mL per liter of total tank water and mix/agitate to fully dissolve (wait a couple of minutes), then adjust pH.
c) add mix A at 10 mL per liter of total tank water and mix/agitate to fully dissolve (wait a couple of minutes).
d) add mix B at 10 mL per liter of total tank water and mix/agitate to fully dissolve (wait a couple of minutes).
e) adjust pH