-
ICMag with help from Landrace Warden and The Vault is running a NEW contest in November! You can check it here. Prizes are seeds & forum premium access. Come join in!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
DIY Nutrients formulations, recipes, chemistry etc.
- Thread starter funkymonkey
- Start date
P
poipu79
Hey Popiu,
Your chart looks good, albeit I find it a bit confusing, or maybe just lacking in explanation. I see my name is part of the file-name, and your ppm profile is close to what I use/suggest. Have you have tested that mix you posted?
I'm glad you're mostly sold on the new ratios and such, I'm looking forward to reading about your experience using your fertilizer solution(s).
@ 1971,
I haven't worked on Hydrobuddy this week, I've been too busy designing my Aerated Deep Flow Technique (ADFT) water culture system. I will get back to Hydrobuddy next week, and post my other mixes next week.
If I had more hours per day, or if I was better at managing my time, I could get a lot more done
I haven't worked on Hydrobuddy this week, I've been too busy designing my Aerated Deep Flow Technique (ADFT) water culture system. I will get back to Hydrobuddy next week, and post my other mixes next week.
If I had more hours per day, or if I was better at managing my time, I could get a lot more done
P
poipu79
hey spurr...old school 100 100 200 grower here. but with an open mind
150 50 200 is my new goal,unused yet
the six pack refers to the five common salts plus micro package available at many hydro stores in .ca and no havenot tried the mix...the 4 part spurr full meal deal including the DAP,CAN,MgNO3 is next

last time I had not calc the ppm of my PK spike ,and I hit ec2.7 for 24 hrs 3 times over a 15 day period ...had to be 500 ppm K
last was a bumper crop
enjoy reading your posts and folowing the various links daily
old nute calc

...poipu
150 50 200 is my new goal,unused yet
the six pack refers to the five common salts plus micro package available at many hydro stores in .ca and no havenot tried the mix...the 4 part spurr full meal deal including the DAP,CAN,MgNO3 is next
last time I had not calc the ppm of my PK spike ,and I hit ec2.7 for 24 hrs 3 times over a 15 day period ...had to be 500 ppm K
last was a bumper crop
enjoy reading your posts and folowing the various links daily
old nute calc
...poipu
The only solubility problem I see would be with the Potassium Nitrate and it would probably only be an issue with a concentrate of around 600X or more. Still I should split the Potassium Nitrate between parts A and Parts B at a concentration of 100X so as to get the EC of Part A close to the EC of Part B (Hydrobuddy software results do not show this split of the Potassium Nitrate between Part A and Part B).
What you need to do, is to make a second entry for KNO3, but use a different label than what comes with HydroBuddy, ex,. use "Potassium Nirate-B". When you make the second entry, make sure to set the "Concentrated Type" to the letter "B", if the default Potassium Nitrate is set to "A".
I just tested such a setup, using the Chilli formula included with Hydrobuddy, here is the result, just what you are looking for, I think:
I use source water at zero ppm and would add the required amount sodium of sodium phosphate to bring the source water to 40 ppm. This water will be added to the diluted nutrient reservoir when ever 1.25 to 2.0 gallons of the diluted reservoir has been used. A Masterflex C/L running two tubes will add both Part A and Part B at the same time very slowly from the concentrate reservoirs. The entry points into the diluted 35 gallon nutrient reservoirs are opposite sides of the reservoir tank. The tank is conical. It is kept aerated with two submersible pumps located just behind the concentrated nutrient entry points. One pumps discharge is pointed downward and the other horizontal and just below the surface. The Masterflex C/L pump dispenses the concentrated Part A and Part B each at only 0.25 ml per minute so there has never been a precipitation issue even using 500X concentrates.
That's a great feature, neat.
The addition of the nutrients by the C/L pump is controlled by an Oakton EC analyzer. I would use the same type analyzer for maintaining the EC of the source water with potassium silicate pumped with a single tube C/L pump at a rate of 0.5 ml per minute. The source water tank is kept filled by a L/S Masterflex pump activated by a Crouzet Pump Down Relay controller and that water is pumped to the reservoir tank by a L/S Masterflex pump activated by another Crouzet Pump Down Relay controller. The pH of the diluted nutrient reservoir can be controlled by a Hach pH analyzer and two C/L pumps with single tubes pumped at 0.05 ml per minute. I have never had to use pH adjustment however as in the past my nutrients in a diluted form have nearly always run between 5.6 and 5.8 as the nutrient system is DTW. I don't mind a bit higher pH but I do not want a lower pH. Nothing better than automation except over zealous dedication of time to a grows daily operation. I just don't have that much time to dedicate to daily operations. That and I am too old and tired to carry around all the water even if I could devote that much time to do everything manually.
Quite a setup you have there

I do not have ready access to Peters Pro Hydro-sol where I live so I would not be adding it to the substances list so my finished weights will be different proportionally than yours but will provide the same ppms in the diluted product as yours mixed in a reservoir with 100 liters of water.
Great, I think (hope) you will be very happy with the results. Let us know how it goes!
One thing of note though. My not using Peter's Hydro-Sol or the epson salts used by you but instead using common magnesium sulfate gave me a sulfur ppm of only 56.9
Interesting. Using the Hydro-Sol must effect the final S ppm, in terms of gross error which is why my S ppm is above what I ideally use (50-60 ppm). Hummm, I really have to dig into HydroBuddy internals next week.
hey spurr...old school 100 100 200 grower here. but with an open mind
150 50 200 is my new goal,unused yet
the six pack refers to the five common salts plus micro package available at many hydro stores in .ca and no havenot tried the mix...the 4 part spurr full meal deal including the DAP,CAN,MgNO3 is next

last time I had not calc the ppm of my PK spike ,and I hit ec2.7 for 24 hrs 3 times over a 15 day period ...had to be 500 ppm K
last was a bumper crop
enjoy reading your posts and folowing the various links daily
old nute calc

...poipu
Great, I hope you like the results. Ex., I think the lower P will impress you during pre-flowering.
P.S. I love your "old nute calc"!
P.P.S. You may want to give HydroBuddy a try, it's quite feature rich, easy to use and bug free (but does have some limitations).
dgr
Member
poipu, are you saying you fed upwards of 500 ppm K for an entire day, three different times? And you believe this resulted in a noticeable increase in yield? What part of the flower cycle was this 15 day period? What was your source of K?last time I had not calc the ppm of my PK spike ,and I hit ec2.7 for 24 hrs 3 times over a 15 day period ...had to be 500 ppm K
last was a bumper crop
Thank you
canna sell all the chemise separate called canna mono nutrients , not sure if this helps any of you guys but thought if you are finding some types of chems hard to find then it may help at least with tests ,
also i had a quick read of the thread . i noticed you guys were talking about a better quality base chems that the hydro market dont use , so the canna monos may not be any use to you .
just thought i would throw it in just in case .
great thread guys , i used to make my own chems a long time ago ,not in this detail but they worked fine . i used chempack salts
also i had a quick read of the thread . i noticed you guys were talking about a better quality base chems that the hydro market dont use , so the canna monos may not be any use to you .
just thought i would throw it in just in case .
great thread guys , i used to make my own chems a long time ago ,not in this detail but they worked fine . i used chempack salts
Y
YosemiteSam
Straight up fuckin killin it with this formula in coco veg:
cano3...3 in grams per gallon
mgso4...2.8
kno3...0.7
ksil...0.7
mkp...0.4
micro...0.1 STEM
150-24-150-144-70-100 N-P-K-Ca-Mg-SiO2
Plus my water has 20 ppm Ca.
I will add K and possibly a little more P for flower.
cano3...3 in grams per gallon
mgso4...2.8
kno3...0.7
ksil...0.7
mkp...0.4
micro...0.1 STEM
150-24-150-144-70-100 N-P-K-Ca-Mg-SiO2
Plus my water has 20 ppm Ca.
I will add K and possibly a little more P for flower.
P
poipu79
hey dgr... yes, yes, day 18,25,and 32 of 12/12poipu, are you saying you fed upwards of 500 ppm K for an entire day, three different times? And you believe this resulted in a noticeable increase in yie
ld? What part of the flower cycle was this 15 day period? What was your source of K?
Thank you
above was last,below next...maybe
...poipu
@ 1971,
I like using screen shots because I think they are more readable and legible to people, myself included. I can pack in many more data in a screen shot, and still make it legible (ex., spreadsheet), than in a post; ex., by use of tables, graphs, etc.
Here is the same mix as above, but it's made for direct addition to 100 liters, not shown is boric acid at 0.095 grams:
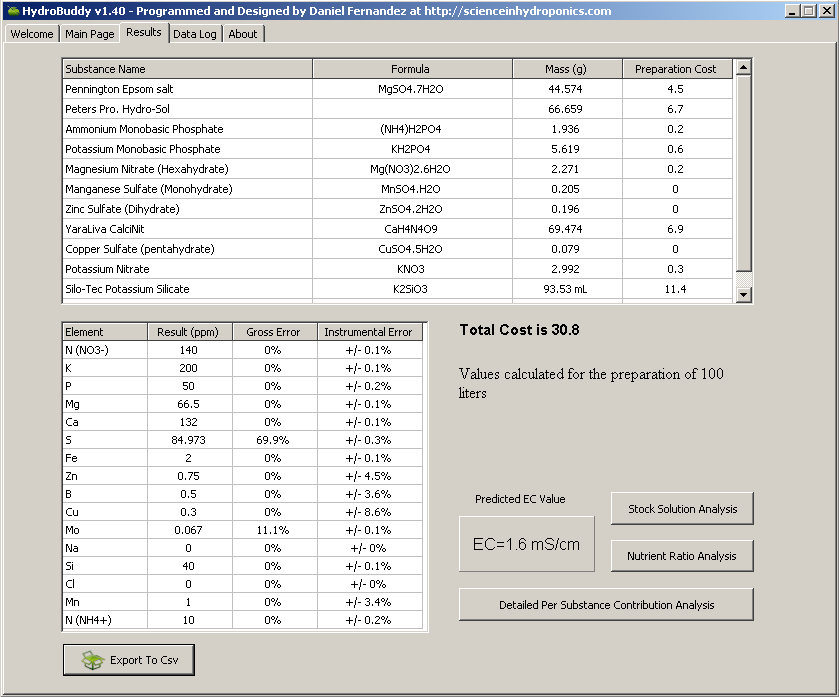
Not gonna lie, a lot of this stuff is going over my head due to no chemistry background/the complexity of what you guys are talking about..
But a few quick questions.
1) So this is a mixture that you would just add directly to a hundred liter reservoir (50 liters, add everything, other 50 liters)?
2) This can be used for the entire cycle of the plant's life? Although it appears as if you will be showing off some new mixes for different stages.
3) Assuming 1 and 2 are correct, how would you go about applying this formula in a recirculating system? Complete reservoir changes at a certain interval (one week? couple days?) Or would you do add-backs?
4) I know I have seen you mention in multiple places plant tissue analysis that has led you to believe in new ratios, can you explain a little more about these? (I have a feeling this will go way over my head once again, but I will do my best to understand)
I apologize for the simple questions, doing my best to try and catch up to this level of knowledge, but I know its going to take time to get this scientific..
it is easier for me to mix up each salt in hot water and then add it to the reservoir. less chance of precipitation.
i believe it could be, but there might be reasons to make adjustments based on the strain.
i change about every week or so and just add ph corrected water. not sure what others do
i believe it could be, but there might be reasons to make adjustments based on the strain.
i change about every week or so and just add ph corrected water. not sure what others do
so i'm trying out the formula spurr listed, and it is treating me really well so far. you don't need to have a couple nutrient profiles like early flowering, late flowering, etc. but it might squeeze a little extra out of your plants. you will never truly know, but if it makes you think so, go for it.
there is a learning curve if you choose to try and understand what the plants do with each nutrient, etc.
i did have an issue trying to learn what salts work best, and there is really no right answer... it depends on if you use direct addition versus A/B solutions. i use direct addition which is easier, and so the salts i've picked are going to be different than what spurr listed for his formula.
there is a learning curve if you choose to try and understand what the plants do with each nutrient, etc.
i did have an issue trying to learn what salts work best, and there is really no right answer... it depends on if you use direct addition versus A/B solutions. i use direct addition which is easier, and so the salts i've picked are going to be different than what spurr listed for his formula.
hiya, Ah man it sucks that this thread is dying ! was enjoying it so so much , realy learned alot in here ! thanks Spurr and others , realy sad that Spurr is not posting anymore ( man u are a information central dude haha).. Thanks for all the info Spurr , your posts are very good and has inspired me a great deal . thanks!
P
poipu79
hiya, Ah man it sucks that this thread is dying ! was enjoying it so so much , realy learned alot in here ! thanks Spurr and others , realy sad that Spurr is not posting anymore ( man u are a information central dude haha).. Thanks for all the info Spurr , your posts are very good and has inspired me a great deal . thanks!


