-
ICMag with help from Landrace Warden and The Vault is running a NEW contest in November! You can check it here. Prizes are seeds & forum premium access. Come join in!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
DIY Nutrients formulations, recipes, chemistry etc.
- Thread starter funkymonkey
- Start date
The mix isn't defined by the relative values for N-P-K or K-Ca-Mg (with respect to ppm). That's just a way to quantify relative amount of ions to each other with respect to effect on plant growth. N-P-K and K-Ca-Mg (relative to ppm) is like the NO3:NH4 ratio, or the P:S ratio, or the Fe:Mn ratio. And it makes comparisons easier.
When it comes to lowering or rising a cation such as Ca or K, knowing the relative amount of other ions that affect its uptake (ex., K-Ca-Mg) is important. That way one does not change that relative amount when changing the concentration of single cation or anion (ex., NO3:S). This has to due to uptake of cations and anions, etc.
I think the best way to define a mix, is by ppm per element. And EC follows, one can find approximate EC from the salts used. I define (qualify and quantify) all my mixes by ppm per element. You can find those data in the screen caps I posted, for direct addition and use of stock solutions (the final ppms are the same, either way)
When it comes to lowering or rising a cation such as Ca or K, knowing the relative amount of other ions that affect its uptake (ex., K-Ca-Mg) is important. That way one does not change that relative amount when changing the concentration of single cation or anion (ex., NO3:S). This has to due to uptake of cations and anions, etc.
I think the best way to define a mix, is by ppm per element. And EC follows, one can find approximate EC from the salts used. I define (qualify and quantify) all my mixes by ppm per element. You can find those data in the screen caps I posted, for direct addition and use of stock solutions (the final ppms are the same, either way)
Spurr,
Are you sure your calculations for the Si ppm are correct?
I'm unable to make sense of it.
If Silo-tec is 7.5% SiO2, then it is 3.506% Si.(7.5 X .4675)
with out taking the density into consideration, which will change it, but not that much:
3.506 X 10 = 35.06ppm from one mL/Liter
35.06 X 3.11 = 109 ppm Si
Its true I don't have my goggles on, but something doesn't jive.
Are you sure your calculations for the Si ppm are correct?
I'm unable to make sense of it.
If Silo-tec is 7.5% SiO2, then it is 3.506% Si.(7.5 X .4675)
with out taking the density into consideration, which will change it, but not that much:
3.506 X 10 = 35.06ppm from one mL/Liter
35.06 X 3.11 = 109 ppm Si
Its true I don't have my goggles on, but something doesn't jive.
Hey Avenger,
No, your goggles are working fine. Thak you very much for double checking what you did, you found something I should have noticed right away; thanks
I hadn't found Si by hand yet for Silo-Tec, I still have to double check all those calculations of HydroBuddy, but besides Si they should be accurate (unless I messed up somewhere). Those calculations for Silo-Tec are using specific gravity of 1.22, a rough calculation I made from g/mL, not knowing precisely the weight of 2.5 gallons.
Okay, I see the problem, for some reason I entered 30% purity for Silo-Tec, I must typed that by mistake. Also, for some reason HydroBuddy is converting 7.5% SiO2 into 3.513% Si; odd. Not only that, but I set K to 3%, not the 2.4903% it really should be, i.e., K2O was 3%. I worked on this late at night a few days ago, I think I entered data while sleepy ... oops. I fixed the Silo-Tec data so Si is now 3.5055%, K is 2.4903% and purity is 100% (because I don't know the actual purity).
I'm glad you found that error and pointed it out, thanks! It lowered cost a good deal, with much less Silo-Tec being needed.
After correcting the 30% purity error, Si and K errors for Silo-Tec, I doubled checked the math for direct addition to 100 liter to achieve 40 ppm Si. Hydrobuddy tells me I need 93.53 mL Silo-Tec, in 100 liters to provide 40 ppm. Double checking that math ...

No, your goggles are working fine. Thak you very much for double checking what you did, you found something I should have noticed right away; thanks
I hadn't found Si by hand yet for Silo-Tec, I still have to double check all those calculations of HydroBuddy, but besides Si they should be accurate (unless I messed up somewhere). Those calculations for Silo-Tec are using specific gravity of 1.22, a rough calculation I made from g/mL, not knowing precisely the weight of 2.5 gallons.
Okay, I see the problem, for some reason I entered 30% purity for Silo-Tec, I must typed that by mistake. Also, for some reason HydroBuddy is converting 7.5% SiO2 into 3.513% Si; odd. Not only that, but I set K to 3%, not the 2.4903% it really should be, i.e., K2O was 3%. I worked on this late at night a few days ago, I think I entered data while sleepy ... oops. I fixed the Silo-Tec data so Si is now 3.5055%, K is 2.4903% and purity is 100% (because I don't know the actual purity).
I'm glad you found that error and pointed it out, thanks! It lowered cost a good deal, with much less Silo-Tec being needed.
After correcting the 30% purity error, Si and K errors for Silo-Tec, I doubled checked the math for direct addition to 100 liter to achieve 40 ppm Si. Hydrobuddy tells me I need 93.53 mL Silo-Tec, in 100 liters to provide 40 ppm. Double checking that math ...
Silo-Tec has SP of ~1.22, 3% K2O and 7.5% SiO2. So, 7.5% SiO2 times 46.74% (Si within SiO2) = 3.5055% Si by weight in Silo-Tec
I updated my screen shots in the posts on the previous page, they are now corrected for the errors you found. The weights should now be accurate for stock solutions and direct addition; however, I will double check all of HydroBuddy's math tomorrow.- (1.22)93.53 = 114.1066
- (114.1066)0.035055 = 4.000006863
- 4/100,000 = 4E^-5
- (4E^-5)1,000,000 = 40 ppm Si

Last edited:
wouldn't it be quicker to just provide n-p-k-mg-ca values versus doing screen shots?
i do direct addition so it is a bit easier versus making concentrates.
Here ya go, for 100 liters:
(it's late here, so I'll double check this in the morning)
Pennington Epsom salt = 44.574 grams
Peter's Pro. Hydro-Sol = 66.659 grams
mono ammonium phosphate = 1.936 grams
mono potassium phosphate = 5.619 grams
magnesium nitrate = 2.271 grams
manganese sulfate = 0.205 grams
zinc sulfate = 0.196 grams
YaraLiva Calcinit = 69.474 grams
copper sulfate = 0.079 grams
potassium nitrate = 2.992 grams
Silo-Tec = 93.53 mL
boric acid = 0.095 grams
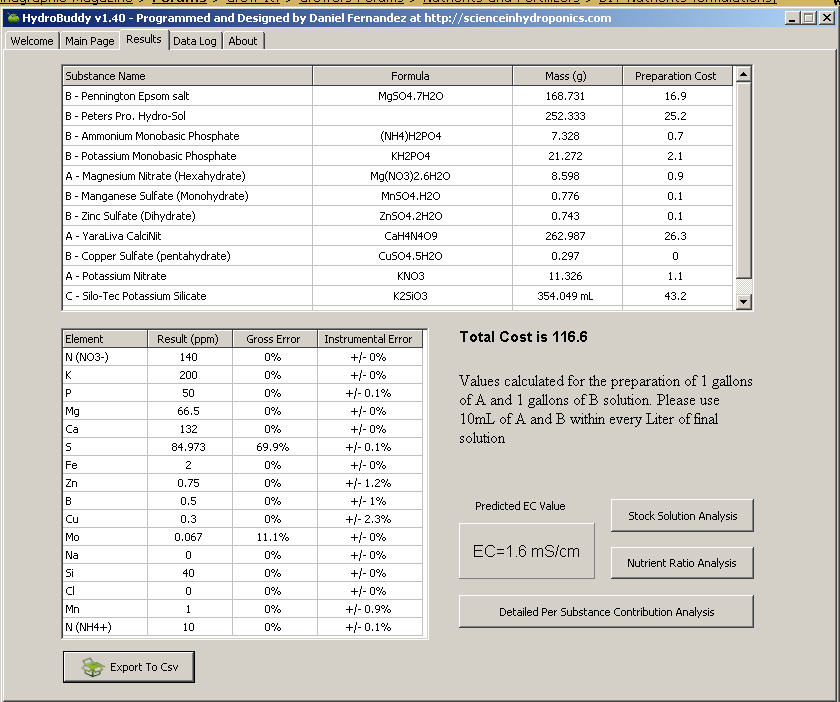
Gives an approx. EC of 1.6 mS/cm, and a ppm profile of:
total N (ammonicial + nitrate) = 150 ppm
NO3 = 140 ppm
NH4 = 10 ppm
P = 50 ppm
K = 200 ppm
Ca = 132 ppm
Mg = 66.5 ppm
S = ~70-80 ppm
Si = 40 ppm
Fe = 2 ppm
Mn = 1 ppm
B = 0.5 ppm
Zn = 0.75 ppm
Cu = 0.3 ppm
Mo = 0.067 ppm
Last edited:
dgr
Member
spurr,
Thanks for posting up more foo on custom mixing. I appreciate you putting it all into a post or two. Specifically the NPK and KCaMg ratios.
I should have read ahead before running my head in circles on the silicate. You might want to modify your provided instructions where you say to "add 10 ml per liter of final volume." It seems to be about 10X too high.
Thanks for posting up more foo on custom mixing. I appreciate you putting it all into a post or two. Specifically the NPK and KCaMg ratios.
I should have read ahead before running my head in circles on the silicate. You might want to modify your provided instructions where you say to "add 10 ml per liter of final volume." It seems to be about 10X too high.
spurr,
Thanks for posting up more foo on custom mixing. I appreciate you putting it all into a post or two. Specifically the NPK and KCaMg ratios.
You're welcome.
I should have read ahead before running my head in circles on the silicate. You might want to modify your provided instructions where you say to "add 10 ml per liter of final volume." It seems to be about 10X too high.
Why do you think that is incorrect? I do not know the exact directions to which you are referring.
After making mix C (stock solution of Silo-Tec; potassium silicate), one would use 10 mL of mix C per liter of fertigation water. Ex., if you have 1 gallon of fertigation water, you would use 37.85 mL of mix C, because there are 3.785 liters per gallon.
However, making stock solution of a liquid potassium silicate product seems a bit redundant. I included the info for the sake of thoroughness and in case someone wished to do so.
T
thefatman
Spurr
Have you tried to formulate this on Hydrobuddy for a two part concentrate without the silica as I doubt that it can be added at that strength in a concentrate. I can't seem to get Hydrobuddy to make a 500X concentrate. I want a gallon of part A and a gallon of Part B but no matter how I input it in Hydrobuddy it gets totally off the wall output. I do not mix and store diluted nutrients so I need a 2 part concentrate. As usual I can not get Nutron2000 to formulate a mix with magnesium nitrate. I do not want to have to set Nutron200o locked at 132 ppm and then calculate manually to 140 ppm of nitrogen, then add the mag sulfate after calculating the magnesium contribution from the magnesium nitrate. I can deal with a larger nitrate and less ammonium content as it will be drain to waste. I can add the silo-tec to the water not the concentrate stock.
Have you played with the two part concentrate feature on Hydrobuddy yet?
Have you tried to formulate this on Hydrobuddy for a two part concentrate without the silica as I doubt that it can be added at that strength in a concentrate. I can't seem to get Hydrobuddy to make a 500X concentrate. I want a gallon of part A and a gallon of Part B but no matter how I input it in Hydrobuddy it gets totally off the wall output. I do not mix and store diluted nutrients so I need a 2 part concentrate. As usual I can not get Nutron2000 to formulate a mix with magnesium nitrate. I do not want to have to set Nutron200o locked at 132 ppm and then calculate manually to 140 ppm of nitrogen, then add the mag sulfate after calculating the magnesium contribution from the magnesium nitrate. I can deal with a larger nitrate and less ammonium content as it will be drain to waste. I can add the silo-tec to the water not the concentrate stock.
Have you played with the two part concentrate feature on Hydrobuddy yet?
Spurr
Have you tried to formulate this on Hydrobuddy for a two part concentrate without the silica as I doubt that it can be added at that strength in a concentrate.
Yes, I made 3 part concentrates. Part A and B are normal (part A having CaNO3 and MgNO3 for example), and part C is only potassium silicate. Because the potassium silicate product the person who hired me uses is liquid (Silo-Tec), I used liquid in my mixes I posted on page 13 and 14. The only reason I made a part C, was so addition of potassium silicate would be the same 10 mL/liter as part A and B.
I can't seem to get Hydrobuddy to make a 500X concentrate. I want a gallon of part A and a gallon of Part B but no matter how I input it in Hydrobuddy it gets totally off the wall output. I do not mix and store diluted nutrients so I need a 2 part concentrate.
Why not use a 'concentration factor' of 100 (i.e., 100x)? As that's the normal ratio for fertilizer stock solutions, ex., most fertilizer injector uses a 1:100 ratio. Stock solutions for other substances like PGRs, are often 1000x, but for fertilizer I think 100x works well. Do you not think so?
As usual I can not get Nutron2000 to formulate a mix with magnesium nitrate. I do not want to have to set Nutron200o locked at 132 ppm and then calculate manually to 140 ppm of nitrogen, then add the mag sulfate after calculating the magnesium contribution from the magnesium nitrate. I can deal with a larger nitrate and less ammonium content as it will be drain to waste. I can add the silo-tec to the water not the concentrate stock.
Have you played with the two part concentrate feature on Hydrobuddy yet?
Yes:
Spurr wrote:
I was hired recently to create a few cannabis specific fertilizer formulations for various stages of growth, EC, source water, etc. Initially the person that hired me asked me to use Peter's Professional Hydro-Sol (5-11-26) as a base.
Below are some excerpts from something I wrote about this mix and making stock solutions in general, etc. I used HydroBuddy to make the following mix, and I am posting a version that assumes pure source water (i.e., doesn't account for ions as EC or elements, in the source water). I also didn't account for P from phosphoric acid, a common pH down product.
Below is a screen shot that lists the salt compounds used, not shown is 0.361 grams of boric acid into mix B. And how to mix bottles A, B and C. Use of A, B and C is 10 mL per liter of fertigation water, each.
The person that hired me grows in Sun Shine Mix #4 using drain-to-waste on a drip ring and timer; flushed weekly with plain water.
I.) Notes:
...
5) After messing around with lots of mixes, I found the perfect mix of inexpensive salts. The mix provides N-P-K of 3-1-4 and K-Ca-Mg of 3.2-1.84-1 [i.e., 4-3.2-1.25, what you requested]; as well as providing sufficient Si, NO3:NH4 ratio of 14, etc. The following are the salts I used, including those you don't yet have. Using the following salts, excluding the micro's, increase the accuracy of the ppm calculations a great deal, which is a very good thing. Using all of the following salts I was able to reduce the price per unit, too:
...
--> I attached two screen shots, the first one, "complete_mix_a", shows how to make the stock solutions (mixes) A, B and C. Ex., how much of each compound to add to each gallon to make stock solution (not shown is 0.361 grams of boric acid into mix B). That screen shot also shows the elemental profile by ppm, providing exactly what you asked for. The EC shown is 1.6 mS/cm, which is the EC of the fertagation water after the specified volumes of mix A, B and C are added. If we include the EC of your source water, i.e., 0.4 mS/cm, the total EC for your fertigtion tank will be 2.0 mS/cm; and I believe that is the EC you requested, correct?
II.) Mixing notes for stock solution:
a) when weighing dry salts use two scales: one that measures down to 0.001 gram and is accurate to 0.001 gram (jewelry scale for micro-nutrients), and one that measures to 1 kilogram and accurate to 0.01 gram, better yet 0.001 gram. Following is an example of en Ebay search for 0.001 gram scales: http://shop.ebay.com/items/?_nkw=sc...pt=1&_sadis=&LH_CAds=&clk_rvr_id=248929254212
b) when measuring liquid volume, ex., for Silo-Tec potassium silicate, use a good graduated cylinder, and a syringe for single mL and less than mL measurements. However, we can find the specific gravity of Silo-Tec and then use weight, instead of volume, to measure out Silo-Tec for mix C.
c) fill jug for mix A with 1/2 gallon distilled or deionized water, then add the salt compounds for mix A, shake and dissolve with each addition. Then add the other 1/2 gallon distilled or deionized water and shake.
d) do the same as above (re: mix A), when mixing B, and C.
III.) Mixing notes for fertigation tank (working solution):
a) fill tank to 1/2 volume with source water needed, ex., if you need 100 liter, fill with 50 liter.
b) add mix C (potassium silicate) at 10 mL per liter of total tank water and mix/agitate to fully dissolve (wait a couple of minutes), then adjust pH.
c) add mix A at 10 mL per liter of total tank water and mix/agitate to fully dissolve (wait a couple of minutes).
d) add mix B at 10 mL per liter of total tank water and mix/agitate to fully dissolve (wait a couple of minutes).
e) adjust pH
@ thefatman,
Have you looked into solubility of elements at a concentration factor of 500? In other words, have you done the math to make sure using a concentration factor of 500 will allow for full solubility of all salts into a gallon of water? Also, do you heat the water before adding dry salts?
Good ref. by Smart! Fertilizer Software company: http://www.smart-fertilizer.com/articles/fertilizer-solubility
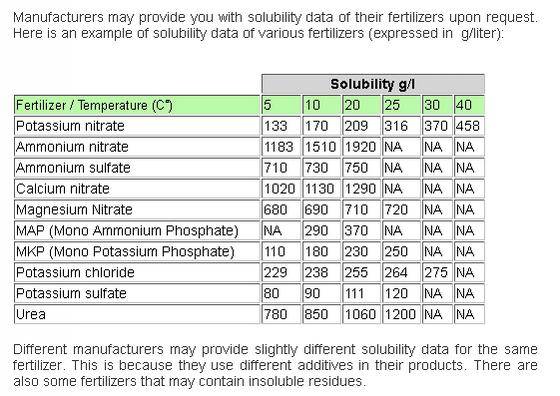
Ps. Here are two more good resources by Smart! Fertilizer Software company:
Have you looked into solubility of elements at a concentration factor of 500? In other words, have you done the math to make sure using a concentration factor of 500 will allow for full solubility of all salts into a gallon of water? Also, do you heat the water before adding dry salts?
Good ref. by Smart! Fertilizer Software company: http://www.smart-fertilizer.com/articles/fertilizer-solubility
Ps. Here are two more good resources by Smart! Fertilizer Software company:
@ all,
I thought this screen shot might be helpful to some, so wrong stock solutions mixes are not made (ex., CaNO3 and MgSO4 in the same concentrate):
Taken from the following article by Smart! Fertilizer Software company:
http://www.smart-fertilizer.com/articles/fertilizer-solubility
Mixing chart:


Jar test:

I thought this screen shot might be helpful to some, so wrong stock solutions mixes are not made (ex., CaNO3 and MgSO4 in the same concentrate):
Taken from the following article by Smart! Fertilizer Software company:
http://www.smart-fertilizer.com/articles/fertilizer-solubility
Mixing chart:

Jar test:
Anti-microbial additive to stock solution:
I have read a few papers on this topic, but Daniel Fernandez wrote a good blog post (here), suggesting use of ~100-300 ppm sodium benzoate. I plan to use 100 ppm and see 'how it goes', I'd rather add less than more. Daniel suggests the reason some stock solutions can support growth of fungi and bacteria, is from carbon due to chelating agents; however, I think it also due to the use of ions for biological process. Keeping stock solutions in cool, dark storage is best.
I have read a few papers on this topic, but Daniel Fernandez wrote a good blog post (here), suggesting use of ~100-300 ppm sodium benzoate. I plan to use 100 ppm and see 'how it goes', I'd rather add less than more. Daniel suggests the reason some stock solutions can support growth of fungi and bacteria, is from carbon due to chelating agents; however, I think it also due to the use of ions for biological process. Keeping stock solutions in cool, dark storage is best.
I wanted to post up a couple of blog post links, to the blog run by Daniel Fernandez (author of Hydrobuddy).
IMO Daniel is a very well educated chemist, and he knows quite a lot about horticulture and hydroponics too. However, some of his claims with respect to plant science and horticulture are not wholly accurate. If I post such blog posts by Daniel, I will add what I think are caveats to some claims he made.

IMO Daniel is a very well educated chemist, and he knows quite a lot about horticulture and hydroponics too. However, some of his claims with respect to plant science and horticulture are not wholly accurate. If I post such blog posts by Daniel, I will add what I think are caveats to some claims he made.
"Understanding Reagent Purity and Its Importance in Hydroponics"
http://scienceinhydroponics.com/201...purity-and-its-importance-in-hydroponics.html
"Instrument Precision: Its Importance in the Preparation of Hydroponic Nutrient Solutions"
http://scienceinhydroponics.com/201...tance-in-hydroponic-solution-preparation.html
http://scienceinhydroponics.com/201...purity-and-its-importance-in-hydroponics.html
"Instrument Precision: Its Importance in the Preparation of Hydroponic Nutrient Solutions"
http://scienceinhydroponics.com/201...tance-in-hydroponic-solution-preparation.html

Okay, last post for now.
This post is about my comments in regard to a blog post by Daniel Fernandez:
Ideally source water (for irrigation and fertigation) has 40-60 ppm alkalinity, with outside range of 30-80 ppm; over 100 ppm is too high. IIRC, water can have (nearly) sufficient alkalinity of 20-30 ppm and not be alkaline, the water can still be weakly acidic.
For some time (years) I have been using citric acid and phosphoric acid as pH down; and adding potassium bicarbonate to increase alkalinity and/or pH (if the alkalinity and/or pH was too low). Doing so helps to keep fertigation water pH buffered against wide pH swings, ex., in a recirculating system; and it keeps soilless solution pH buffered against wide pH swings. Citric acid also help keep P anions soluble by complexing/bonding to Fe, Al, etc., and dissociating P-Fe, etc.
Here are few reasons pH swings can happen:
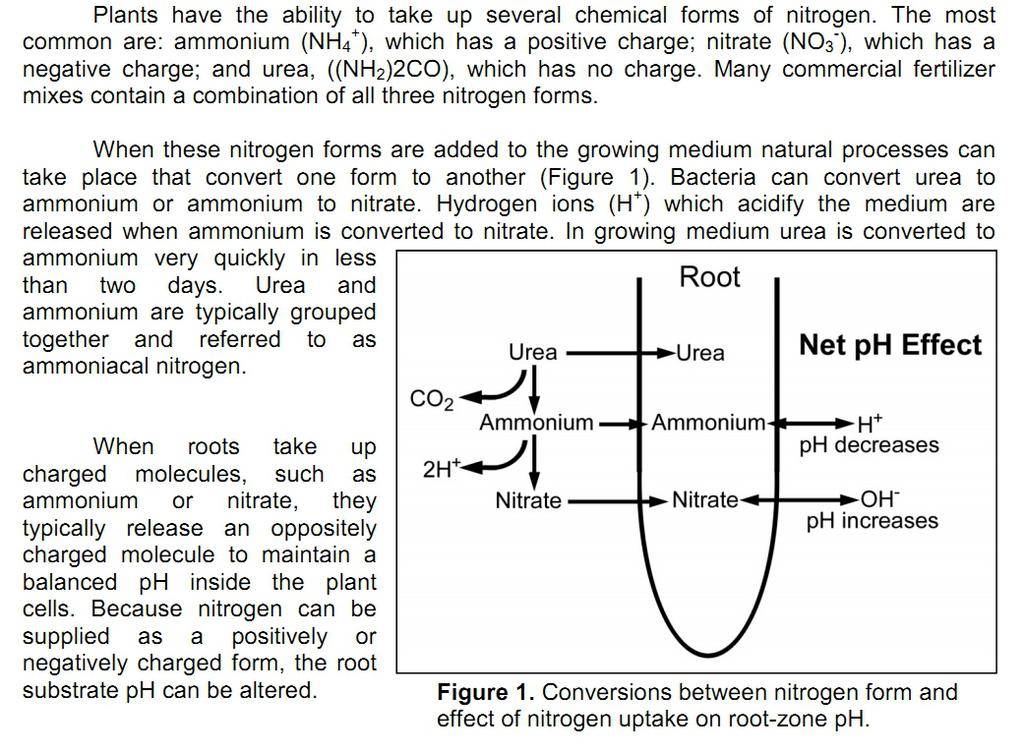
[1] "Nitrogen: All forms are not equal"
Neil Mattson, Roland Leatherwood and Cari Peters
GMPro, June 2009; reprint from Cornell Univeristy Cooperative Extension
www.greenhouse.cornell.edu/crops/factsheets/nitrogen_form.pdf
[2] "Understanding Plant Nutrition: Fertilizers And Media pH"
Bill Argo and Paul Fisher
Greenhouse Grower, July 2008
http://www.greenhousegrower.com/magazine/?storyid=560

This post is about my comments in regard to a blog post by Daniel Fernandez:
"Chemical Buffers in Hydroponics: What is the Best, Cheapest Buffer"
http://scienceinhydroponics.com/201...oponics-what-is-the-best-cheapest-buffer.html
One of the main reasons I use both citric acid and phosphoric acid, besides for pH down and to help keep P anions solute (re citric acid), is to help buffer pH against wide swings. Likewise, I make sure my fertigation water has alkalinity of at least 20 ppm to buffer pH against wide swings. Below, when Daniel refers to carbonates, he is really discussing water alkalinity. I.e., mainly the amount of bicarbonates and carbonates, alkalinity and alkaline are not the same thing. http://scienceinhydroponics.com/201...oponics-what-is-the-best-cheapest-buffer.html
Ideally source water (for irrigation and fertigation) has 40-60 ppm alkalinity, with outside range of 30-80 ppm; over 100 ppm is too high. IIRC, water can have (nearly) sufficient alkalinity of 20-30 ppm and not be alkaline, the water can still be weakly acidic.
For some time (years) I have been using citric acid and phosphoric acid as pH down; and adding potassium bicarbonate to increase alkalinity and/or pH (if the alkalinity and/or pH was too low). Doing so helps to keep fertigation water pH buffered against wide pH swings, ex., in a recirculating system; and it keeps soilless solution pH buffered against wide pH swings. Citric acid also help keep P anions soluble by complexing/bonding to Fe, Al, etc., and dissociating P-Fe, etc.
Here are few reasons pH swings can happen:
- lack of water alkalinity
- exudates from roots due to different types of nitrogen, ex., H+ (pH down) due to ammonium uptake and OH- (pH up) due to nitrate uptake [1,2]
- exudates from roots and microbes, ex., citrate, oxalate, citric acid, etc., as well as other organic acids (pH down)
- "ammonification" and "nitrification" by microbes (pH down and pH up) [1,2]
- release of Co2 by roots, forming "carbonic acid" when bonded to H20 (pH down)
- etc.
References:Daniel Fernandez wrote:
...
The buffers we are left with are then very simple organic and inorganic substances that have low phytotoxicity and some compatibility with the other ions present in our hydroponic setup. From these ions phosphate species, citrate species and carbonates are the most important ones we can use within our hydroponics setup. However we are limited by the actual concentration values of each we can use and for this reason we cannot have unlimited buffering capacity from these sources.
Which one is best? We can actually carry out simulations to show us the pH vs acid-base addition for different hydroponic solution constitutions using mathematical equations. Running these simulations requires the solution of highly complex systems of equations which contain all the information relative to the chemical equilibrium of all the different existing ionic species. The below shown simulations were carried out using the Mathematica computer program (all solutions are assumed to be adjusted to an initial pH of 5.8 with a strong acid or base).

The blue curve represents the behavior of a poorly buffered hydroponic solution with only about 0.002M phosphate concentration (about 50 ppm of P). The red and yellow curves represent two solutions with increasing levels of carbonate showing us that if you are battling pH increases, having more carbonate will definitely help you deal with this. However it is also clear that carbonate concentrations at pH 5.8 are restricted to around 100 ppm since values above this are bound to cause toxicity due to the very large presence of the hydrogen carbonate ion. The green curve represents an increase in the amount of phosphate from 0.002 to 0.004M (about 100 ppm) with carbonate, showing us that phosphates are not good at buffering increases towards the upper side but they do increase buffering towards acid territory. Overall I also noticed that citrate concentration increases to the maximum threshold allowed by calcium citrate solubility did not afford a very good buffering effect with only a mild effect that prevented shifts towards the downside.
In the end, the conclusion seems to be that in a regular hydroponics system where pH increases generally happen towards the upside it is better to use carbonate as a buffering agent than to use citrate or phosphate although phosphate at its regular concentration in hydroponic does provide some buffering against pH moves (without phosphate increases are much more dramatic). For this reason I believe that a phosphate/carbonate buffer seems to be the best choice for most hydroponic growers, taking care to keep the concentrations at levels that do not cause precipitation or phytotoxicity problems.
[1] "Nitrogen: All forms are not equal"
Neil Mattson, Roland Leatherwood and Cari Peters
GMPro, June 2009; reprint from Cornell Univeristy Cooperative Extension
www.greenhouse.cornell.edu/crops/factsheets/nitrogen_form.pdf
Bill Argo and Paul Fisher
Greenhouse Grower, July 2008
http://www.greenhousegrower.com/magazine/?storyid=560

Last edited:
T
thefatman
The only solubility problem I see would be with the Potassium Nitrate and it would probably only be an issue with a concentrate of around 600X or more. Still I should split the Potassium Nitrate between parts A and Parts B at a concentration of 100X so as to get the EC of Part A close to the EC of Part B (Hydrobuddy software results do not show this split of the Potassium Nitrate between Part A and Part B).
I use source water at zero ppm and would add the required amount sodium of sodium phosphate to bring the source water to 40 ppm. This water will be added to the diluted nutrient reservoir when ever 1.25 to 2.0 gallons of the diluted reservoir has been used. A Masterflex C/L running two tubes will add both Part A and Part B at the same time very slowly from the concentrate reservoirs. The entry points into the diluted 35 gallon nutrient reservoirs are opposite sides of the reservoir tank. The tank is conical. It is kept aerated with two submersible pumps located just behind the concentrated nutrient entry points. One pumps discharge is pointed downward and the other horizontal and just below the surface. The Masterflex C/L pump dispenses the concentrated Part A and Part B each at only 0.25 ml per minute so there has never been a precipitation issue even using 500X concentrates. The addition of the nutrients by the C/L pump is controlled by an Oakton EC analyzer. I would use the same type analyzer for maintaining the EC of the source water with potassium silicate pumped with a single tube C/L pump at a rate of 0.5 ml per minute. The source water tank is kept filled by a L/S Masterflex pump activated by a Crouzet Pump Down Relay controller and that water is pumped to the reservoir tank by a L/S Masterflex pump activated by another Crouzet Pump Down Relay controller. The pH of the diluted nutrient reservoir can be controlled by a Hach pH analyzer and two C/L pumps with single tubes pumped at 0.05 ml per minute. I have never had to use pH adjustment however as in the past my nutrients in a diluted form have nearly always run between 5.6 and 5.8 as the nutrient system is DTW. I don't mind a bit higher pH but I do not want a lower pH. Nothing better than automation except over zealous dedication of time to a grows daily operation. I just don't have that much time to dedicate to daily operations. That and I am too old and tired to carry around all the water even if I could devote that much time to do everything manually.
I do not have ready access to Peters Pro Hydro-sol where I live so I would not be adding it to the substances list so my finished weights will be different proportionally than yours but will provide the same ppms in the diluted product as yours mixed in a reservoir with 100 liters of water.
I got the software to provide all the finished data. Farm out!! Beats the hell out having to do so many manual calculations after only getting part of the formulation calculated by the Nutron2000 software.
One thing of note though. My not using Peter's Hydro-Sol or the epson salts used by you but instead using common magnesium sulfate gave me a sulfur ppm of only 56.9
I use source water at zero ppm and would add the required amount sodium of sodium phosphate to bring the source water to 40 ppm. This water will be added to the diluted nutrient reservoir when ever 1.25 to 2.0 gallons of the diluted reservoir has been used. A Masterflex C/L running two tubes will add both Part A and Part B at the same time very slowly from the concentrate reservoirs. The entry points into the diluted 35 gallon nutrient reservoirs are opposite sides of the reservoir tank. The tank is conical. It is kept aerated with two submersible pumps located just behind the concentrated nutrient entry points. One pumps discharge is pointed downward and the other horizontal and just below the surface. The Masterflex C/L pump dispenses the concentrated Part A and Part B each at only 0.25 ml per minute so there has never been a precipitation issue even using 500X concentrates. The addition of the nutrients by the C/L pump is controlled by an Oakton EC analyzer. I would use the same type analyzer for maintaining the EC of the source water with potassium silicate pumped with a single tube C/L pump at a rate of 0.5 ml per minute. The source water tank is kept filled by a L/S Masterflex pump activated by a Crouzet Pump Down Relay controller and that water is pumped to the reservoir tank by a L/S Masterflex pump activated by another Crouzet Pump Down Relay controller. The pH of the diluted nutrient reservoir can be controlled by a Hach pH analyzer and two C/L pumps with single tubes pumped at 0.05 ml per minute. I have never had to use pH adjustment however as in the past my nutrients in a diluted form have nearly always run between 5.6 and 5.8 as the nutrient system is DTW. I don't mind a bit higher pH but I do not want a lower pH. Nothing better than automation except over zealous dedication of time to a grows daily operation. I just don't have that much time to dedicate to daily operations. That and I am too old and tired to carry around all the water even if I could devote that much time to do everything manually.
I do not have ready access to Peters Pro Hydro-sol where I live so I would not be adding it to the substances list so my finished weights will be different proportionally than yours but will provide the same ppms in the diluted product as yours mixed in a reservoir with 100 liters of water.
I got the software to provide all the finished data. Farm out!! Beats the hell out having to do so many manual calculations after only getting part of the formulation calculated by the Nutron2000 software.
One thing of note though. My not using Peter's Hydro-Sol or the epson salts used by you but instead using common magnesium sulfate gave me a sulfur ppm of only 56.9
so i have a question regarding diluting a particular chemical that is required in very small amounts. so hydrobuddy says i need .669g of copper sulfate. if i were to dissolve 66.9g in 1000 ml of water, would that not give me .0669 g per ml? so then i'd only need 10 ml of this concentrated solution to meet my needs... correct?
yes, i am a bonehead and for some reason this really is giving me trouble :/
any help is appreciated
yes, i am a bonehead and for some reason this really is giving me trouble :/
any help is appreciated
dgr
Member
1971,
That is correct. 66.9:1000. 6.69:100. .669:10. Be careful with that. It's a fungicide (good) and it's also used to kill roots.
BTW, I was reading an analysis on the nutrients left in the water and consumed by the plant (it doesn't add to 100%) and a couple of the elements they had greater than 100% return. In other words, they had more of an element at the end than they had put into the solution. Guess which one had a 600% return? They hypothesized that it came from the plastic pipes in their system.
http://www.usu.edu/cpl/research_hydroponics3.htm#recovery
That is correct. 66.9:1000. 6.69:100. .669:10. Be careful with that. It's a fungicide (good) and it's also used to kill roots.
BTW, I was reading an analysis on the nutrients left in the water and consumed by the plant (it doesn't add to 100%) and a couple of the elements they had greater than 100% return. In other words, they had more of an element at the end than they had put into the solution. Guess which one had a 600% return? They hypothesized that it came from the plastic pipes in their system.
http://www.usu.edu/cpl/research_hydroponics3.htm#recovery


