-
As of today ICMag has his own Discord server. In this Discord server you can chat, talk with eachother, listen to music, share stories and pictures...and much more. Join now and let's grow together! Join ICMag Discord here! More details in this thread here: here.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
acespicoli
Well-known member
The most purple black stem astringent noxious weed smell the one you will toss cause it smells like some kind of poison...

In the cure somehow later once the things settle, becomes a incredible musky skunk.


In the cure somehow later once the things settle, becomes a incredible musky skunk.
The promise holds true to numerous combinations of indica and sativa. Have to pheno hunt and develop the line using the plants with the best combination and effect. Select only for resin and less for structure and other traits. Those can be refined in the future.
Sativa Candy Chunk F15
derived from original hybrid in 2010
Sativa Candy Chunk is a mixture of the seed from these 2 lines in the garden in 2010 with pollination by 2 (OG Kush x Jamaican Jam) x DC) x DC males.
(OG Kush x Jamaican Jam) x DC)x DC
Sour Bubble Bx3 x Sour Bubble bx 4 x DC
(Bubba Kush x Bubblegum) x DC) x DC
Sour Bubble Bx3 x Sour Bubble bx 4 x DC

:::::::Pipeline Farms 2024:::::::
Staying warm this year, but its time to take them down. After the hurricane reminants came spinning through, needed to cut tops because would have been too difficult to pic out all the mold, and losses would have been too great. Plants were ready, and have been happy with the harvest. The...
www.icmag.com
Sativa Candy Chunk F15
derived from original hybrid in 2010
Sativa Candy Chunk is a mixture of the seed from these 2 lines in the garden in 2010 with pollination by 2 (OG Kush x Jamaican Jam) x DC) x DC males.
(OG Kush x Jamaican Jam) x DC)x DC
Sour Bubble Bx3 x Sour Bubble bx 4 x DC
(Bubba Kush x Bubblegum) x DC) x DC
Sour Bubble Bx3 x Sour Bubble bx 4 x DC

Last edited:
acespicoli
Well-known member
Muscone is a macrocyclic ketone, an organic compound that is the primary contributor to the odor of musk. Natural muscone is obtained from musk, a glandular secretion of the musk deer, which has been used in perfumery and medicine for thousands of years. Since obtaining natural musk requires killing the endangered animal, nearly all muscone used in perfumery and for scenting consumer products today is synthetic. It has the characteristic smell of being "musky".
One asymmetric synthesis of (−)-muscone begins with commercially available (+)-citronellal, and forms the 15-membered ring via ring-closing metathesis:[1]

A more recent enantioselective synthesis involves an intramolecular aldol addition/dehydration reaction of a macrocyclic diketone.[2]
The sweat is oily, cloudy, viscous, and originally odorless;[49] it gains odor upon decomposition by bacteria. Because both apocrine glands and sebaceous glands open into the hair follicle, apocrine sweat is mixed with sebum.[41]
Chemical structure and synthesis
The chemical structure of muscone was first elucidated by Leopold Ružička. It is a 15-membered ring ketone with one methyl substituent in the 3-position. It is an oily liquid that is found naturally as the (−)-enantiomer, (R)-3-methylcyclopentadecanone. Muscone has been synthesized as the pure (−)-enantiomer as well as the racemate. It is very slightly soluble in water and miscible with alcohol.One asymmetric synthesis of (−)-muscone begins with commercially available (+)-citronellal, and forms the 15-membered ring via ring-closing metathesis:[1]

A more recent enantioselective synthesis involves an intramolecular aldol addition/dehydration reaction of a macrocyclic diketone.[2]
The sweat is oily, cloudy, viscous, and originally odorless;[49] it gains odor upon decomposition by bacteria. Because both apocrine glands and sebaceous glands open into the hair follicle, apocrine sweat is mixed with sebum.[41]
Last edited:
acespicoli
Well-known member
Body odor can smell sweet, sour, tangy or like onions.
What bacteria causes sweat to smell?
The culprit is an enzyme called C-T lyase, found in the bacterium Staphylococcus hominis, which dwells in human armpits. These bacteria feed on odorless chemicals released in sweat, which the enzyme then converts into thioalcohols—a pungent compound responsible for the offending smell.
 en.wikipedia.org
en.wikipedia.org
Another aspect of bacteria is the generation of body odor. Sweat is odorless however several bacteria may consume it and create byproducts which may be considered putrid by humans (as in contrast to flies, for example, that may find them attractive/appealing). Several examples are:
3-Methylbutanoic acid, also known as β-methylbutyric acid or more commonly isovaleric acid, is a branched-chain alkyl carboxylic acid with the chemical formula (CH3)2CHCH2CO2H. It is classified as a short-chain fatty acid. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. The compound occurs naturally and can be found in many foods, such as cheese, soy milk, and apple juice.
3-Methylbutanoic acid is a minor constituent of the perennial flowering plant valerian (Valeriana officinalis), from which it got its trivial name isovaleric acid: an isomer of valeric acid which shares its unpleasant odor.[2] The dried root of this plant has been used medicinally since antiquity.[3][4] Their chemical identity was first investigated in the 19th century by oxidation of the components of fusel alcohol, which includes the five-carbon amyl alcohols.[5]
What bacteria causes sweat to smell?
The culprit is an enzyme called C-T lyase, found in the bacterium Staphylococcus hominis, which dwells in human armpits. These bacteria feed on odorless chemicals released in sweat, which the enzyme then converts into thioalcohols—a pungent compound responsible for the offending smell.
Staphylococcus hominis - Wikipedia
Another aspect of bacteria is the generation of body odor. Sweat is odorless however several bacteria may consume it and create byproducts which may be considered putrid by humans (as in contrast to flies, for example, that may find them attractive/appealing). Several examples are:
- Propionibacteria in adolescent and adult sebaceous glands can turn its amino acids into propionic acid.[26]
- Staphylococcus epidermidis creates body odor by breaking sweat into isovaleric acid (3-methyl butanoic acid).[27]
- Bacillus subtilis creates strong foot odor.[28]
3-Methylbutanoic acid, also known as β-methylbutyric acid or more commonly isovaleric acid, is a branched-chain alkyl carboxylic acid with the chemical formula (CH3)2CHCH2CO2H. It is classified as a short-chain fatty acid. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. The compound occurs naturally and can be found in many foods, such as cheese, soy milk, and apple juice.
3-Methylbutanoic acid is a minor constituent of the perennial flowering plant valerian (Valeriana officinalis), from which it got its trivial name isovaleric acid: an isomer of valeric acid which shares its unpleasant odor.[2] The dried root of this plant has been used medicinally since antiquity.[3][4] Their chemical identity was first investigated in the 19th century by oxidation of the components of fusel alcohol, which includes the five-carbon amyl alcohols.[5]
Last edited:
subbed
acespicoli
Well-known member
welcome, on insubbed
 glad you stopped by, reminds me to look in on your bag seed grows
glad you stopped by, reminds me to look in on your bag seed grows Found it <
acespicoli
Well-known member
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate (SO2−
4) as terminal electron acceptor, reducing it to hydrogen sulfide (H2S).[1][2] Therefore, these sulfidogenic microorganisms "breathe" sulfate rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration.
Most sulfate-reducing microorganisms can also reduce some other oxidized inorganic sulfur compounds, such as sulfite (SO2−
3), dithionite (S
2O2−
4), thiosulfate (S
2O2−
3), trithionate (S
3O2−
6), tetrathionate (S
4O2−
6), elemental sulfur (S8), and polysulfides (S2−
n). Other than sulfate reduction, some sulfate-reducing microorganisms are also capable of other reactions like disproportionation of sulfur compounds. Depending on the context, "sulfate-reducing microorganisms" can be used in a broader sense (including all species that can reduce any of these sulfur compounds) or in a narrower sense (including only species that reduce sulfate, and excluding strict thiosulfate and sulfur reducers, for example).
Sulfate-reducing microorganisms can be traced back to 3.5 billion years ago and are considered to be among the oldest forms of microbes, having contributed to the sulfur cycle soon after life emerged on Earth.[3]
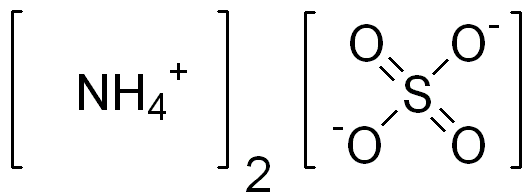
 en.wikipedia.org
en.wikipedia.org

Though bituminous coal varies in its chemical composition, a typical composition is about 84.4% carbon, 5.4% hydrogen, 6.7% oxygen, 1.7% nitrogen, and 1.8% sulfur, on a weight basis.[11]

 en.wikipedia.org
en.wikipedia.org
In North America, where the early Carboniferous beds are primarily marine limestones,
Limestone (calcium carbonate CaCO3)
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.






Lapis of Badakhshan (Beautiful Strains)
Soil chemistry makes a HUGE difference in the terpene production
When growing skunk dont take that lightly
4) as terminal electron acceptor, reducing it to hydrogen sulfide (H2S).[1][2] Therefore, these sulfidogenic microorganisms "breathe" sulfate rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration.
Most sulfate-reducing microorganisms can also reduce some other oxidized inorganic sulfur compounds, such as sulfite (SO2−
3), dithionite (S
2O2−
4), thiosulfate (S
2O2−
3), trithionate (S
3O2−
6), tetrathionate (S
4O2−
6), elemental sulfur (S8), and polysulfides (S2−
n). Other than sulfate reduction, some sulfate-reducing microorganisms are also capable of other reactions like disproportionation of sulfur compounds. Depending on the context, "sulfate-reducing microorganisms" can be used in a broader sense (including all species that can reduce any of these sulfur compounds) or in a narrower sense (including only species that reduce sulfate, and excluding strict thiosulfate and sulfur reducers, for example).
Sulfate-reducing microorganisms can be traced back to 3.5 billion years ago and are considered to be among the oldest forms of microbes, having contributed to the sulfur cycle soon after life emerged on Earth.[3]
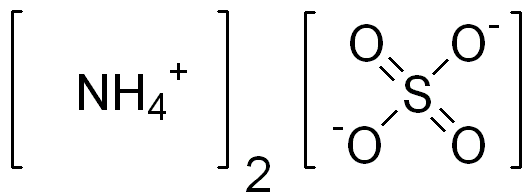
Ammonium sulfate - Wikipedia
Though bituminous coal varies in its chemical composition, a typical composition is about 84.4% carbon, 5.4% hydrogen, 6.7% oxygen, 1.7% nitrogen, and 1.8% sulfur, on a weight basis.[11]

Anthracite - Wikipedia
In North America, where the early Carboniferous beds are primarily marine limestones,
Limestone (calcium carbonate CaCO3)
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.
Lapis of Badakhshan (Beautiful Strains)
Soil chemistry makes a HUGE difference in the terpene production

When growing skunk dont take that lightly

Last edited:
acespicoli
Well-known member
A good source rock for hydrocarbons can contain up to twenty percent organic carbon. Generally, black shale receives its influx of carbon from algae, which decays and forms an ooze known as sapropel. When this ooze is cooked at desired pressure, three to six kilometers (1.8 - 3.7 miles) depth, and temperature, 90–120 °C (194–248 °F), it will form kerogen. Kerogen can be heated, and yield up to 10–150 US gallons (0.038–0.568 m3) of natural oil and gas product per ton of rock.[2]

Mudrock - Wikipedia

Hydrocarbon - Wikipedia
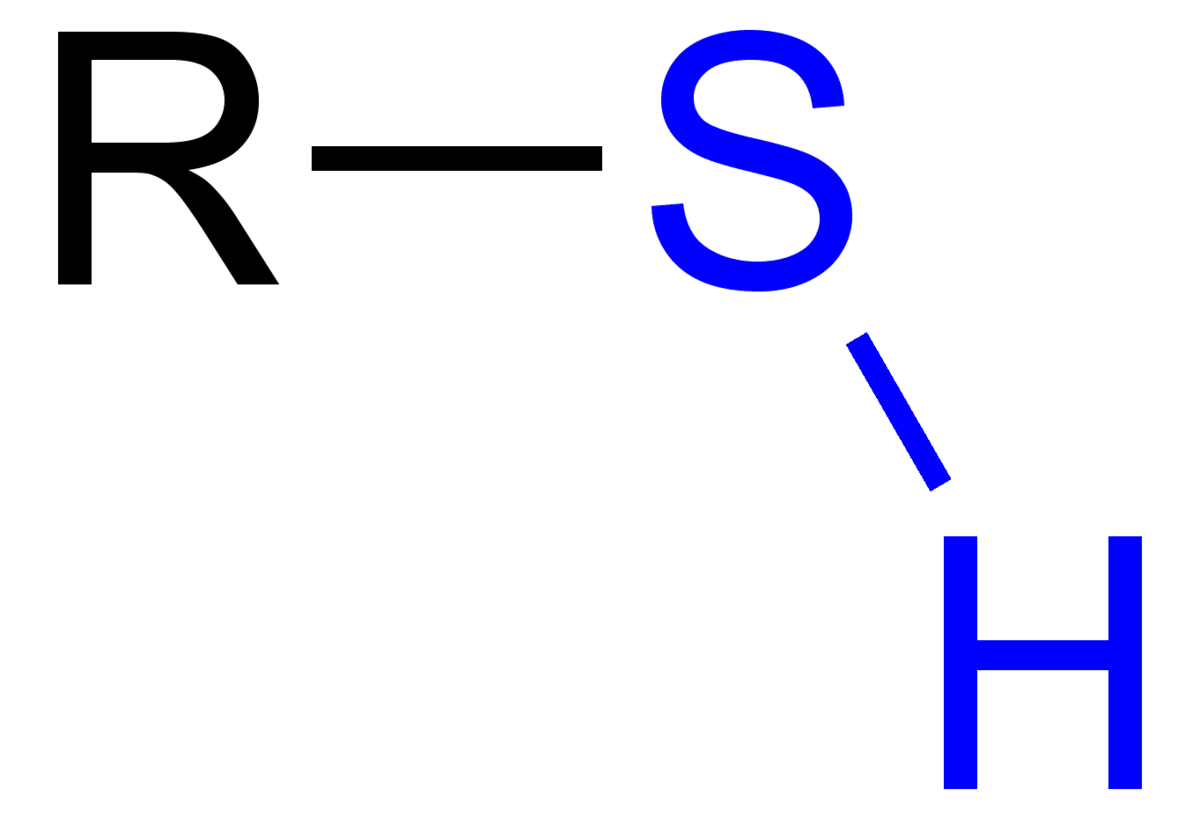
Thiol - Wikipedia

Sesquiterpene - Wikipedia
Last edited:
acespicoli
Well-known member
Nerolidol, also known as peruviol and penetrol , is a naturally occurring sesquiterpene alcohol. A colorless liquid, it is found in the essential oils of many types of plants and flowers.[1] There are four isomers of nerolidol', which differ in the geometry about the central double bond and configuration of the hydroxyl-bearing carbon, but most applications use such a mixture. The aroma of nerolidol is woody and reminiscent of fresh bark. It is used as a flavoring agent and in perfumery as well as in non-cosmetic products such as detergents and cleansers.[2] Nerolidyl derivatives include nerolidyl diphosphate[3] and the fragrance nerolidyl acetate.[4]
Significant sources of natural nerolidol is Cabreuva oil and the oil of Dalbergia parviflora.[4] It is also present in neroli, ginger, jasmine, lavender, tea tree, Cannabis sativa, and lemon grass, and is a dominant scent compound in Brassavola nodosa.[5]
Over 200 species of plants produce linalool, notably from the families Lamiaceae (mint and other herbs), Lauraceae (laurels, cinnamon, rosewood), and Rutaceae (citrus fruits), but also birch trees and other plants, from tropical to boreal climate zones.
Farnesol is a natural pesticide for mites and is a pheromone for several other insects.
In a 1994 report released by five top cigarette companies, farnesol was listed as one of 599 additives to cigarettes.[1] It is a flavoring ingredient.

 en.wikipedia.org
&
en.wikipedia.org
&

 en.wikipedia.org
Linked
en.wikipedia.org
Linked
Farnesol is present in many essential oils such as citronella, neroli, cyclamen, lemon grass, tuberose, rose, musk, balsam, and tolu.
Synthesis and occurrence
Nerolidol is produced commercially from geranylacetone by the addition of vinyl Grignard reagent. It is used as a source of farnesol, vitamin E, and vitamin K1.[4]Significant sources of natural nerolidol is Cabreuva oil and the oil of Dalbergia parviflora.[4] It is also present in neroli, ginger, jasmine, lavender, tea tree, Cannabis sativa, and lemon grass, and is a dominant scent compound in Brassavola nodosa.[5]
See also
Over 200 species of plants produce linalool, notably from the families Lamiaceae (mint and other herbs), Lauraceae (laurels, cinnamon, rosewood), and Rutaceae (citrus fruits), but also birch trees and other plants, from tropical to boreal climate zones.
- Aniba rosaeodora
- Lavandula[6]
- Cinnamomum tamala[7]
- Cannabis sativa[8]
- Basil[9]
- Solidago (goldenrod)[10]
- Artemisia vulgaris (mugwort)
- Humulus lupulus (hop)
Farnesol is a natural pesticide for mites and is a pheromone for several other insects.
In a 1994 report released by five top cigarette companies, farnesol was listed as one of 599 additives to cigarettes.[1] It is a flavoring ingredient.

Musk - Wikipedia

Farnesol - Wikipedia
Farnesol is present in many essential oils such as citronella, neroli, cyclamen, lemon grass, tuberose, rose, musk, balsam, and tolu.
acespicoli
Well-known member
The colas are produced as a flavour concentrate or syrup that is then mixed with bulk ingredients to produce the drink. Completed flavour concentrates are sold by some of the open cola producers.[5] The bulk ingredients include those such as sweeteners (sugar or artificial), caffeine and the source of acidity, phosphoric or citric acid. As these are added later, after the flavour blending stage, sugar and caffeine levels per batch can be tailored to a market's particular taste.
Coca-Cola's own flavouring syrup is known in-house as "Merchandise 7X", which is cross-referenced in the open recipes.[2]
A typical recipe is based on eight essential oils, listed here in approximate order of decreasing volume:[2]

Open-source cola - Wikipedia
NEED INFO ON AE77CALIO PLANTS
hey peeps ive got some ae77calio plants that are 2 weeks in flower at 3 ft & they are all female?? are these fem seeds as it didnt say so on the package i got from a breeder friend of mine... this is one of the few strains that i dont know the complete background on so can someone tell me...
www.icmag.com
The difference between Pepsi and Coke? phosphoric or citric acid
acespicoli
Well-known member
California Orange
breed by The Seed BankHere you can find all info about California Orange from The Seed Bank. If you are searching for information about California Orange from The Seed Bank, check out our: Basic infos, Gallery, Degustation, Awards, Strain Reviews, Direct Comparisons, Lineage / Genealogy, Hybrids / Crossbreeds, User comments, for this cannabis variety here at this page and follow the links to get even more information. Or list all California Orange Strains (± 5) If you have any personal experiences with growing or consuming this cannabis variety, please use the upload links to add them to the database!
Basic infos
California Orange is an indica / sativa from The Seed Bank and can be cultivated indoors and outdoors The Seed Bank's California Orange is/was never available as feminized seeds.The Seed Bank Description
This is one of the best-yielding early varieties from northern California, and probably the easiest one to grow. Another harvest festival winner. It is very resinous, with resin extending almost to the end of the large fan leaves. It is vigorous, disease resistant, and potent. The seedlings are very consistent, and it breeds true. Highly recommended.- Responds well to an indoor environment.
- Height 8 to 12 feet.
- Yield up to 2 lbs.
- Harvest mid Sept. in California.
see also cali-o AE77 Tangie
Tangie (aka) (Tangerine Dream from 1995) She has been around for many years now, but for many more she was hidden, until our good friend Crockett pulled her out of his closet to present us with it! The Genetics are Cali-o X Skunk Hybrid then selected to what we have now the Tangie, She grows nice and tall, has a good yield and easily will become your favourite!. She will continue vertical growth until the 5th week so be aware of your space. The Tangie produce very resinous flowers with an unbelievable aroma of citrus, tangerines!! Also she produces some of the best tasting concentrates on earth! Winning every contest she has been entered in 10 out of 10 contests in 8 months!!! Growing the Tangie outside is a must as she finishes late September early October with heavy yields and super sticky flowers. Tangie is also good to SCROG,SOG, she reacts well to topping or the FIM technique as this will produce more of a bush. For best tasting results grow the Tangie in soil.
Clone Only x Crocket's Selection
30% Indica : 70% Sativa
Flowering Time: 9-10 Weeks
Yield: 450-550 g/m2
Orange Velvet is an mostly indica from Unknown or Legendary and can be cultivated indoors and outdoors Unknown or Legendary's Orange Velvet is/was never available as feminized seeds.
Unknown or Legendary Description
This is a local Orange Skunk, used by TGA Subcool...Jill an accomplished hyrdo grower was lucky enough to be gifted with an amazing Orange Skunk. The day we smoked the finished bud we knew we had to outcross it with our Space Queen male.
Orange skunks were popular for a time
acespicoli
Well-known member
There is this:
Then there is what we are after here in this thread the wild and the domesticate

Sesquiterpenes are C15-terpenoids built from three isoprene units. They are found particularly in higher plants and in many other living systems such as marine organisms and fungi. Naturally, they occur as hydrocarbons or in oxygenated forms including lactones, alcohols, acids, aldehydes, and ketones.
Liktor, Erika & Keresztes, Attila & LaVigne, Justin & Streicher, John & Largent-Milnes, Tally. (2021). Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacological Reviews. 73. 98-126. 10.1124/pharmrev.120.000046.
 en.wikipedia.org
en.wikipedia.org
| 1. | Plants usually with a THC/CBD ratio ≥7; terpenoid profile usually lacks sesquiterpene alcohols, fresh aroma often pleasant. Plants ≥ 2 m tall in good habitats; branches flexible, diverging from the shoot at a relatively acute angle (<45° from vertical). Fresh leaves medium green in color; central leaflets narrow (length/width usually >6), lanceolate to linear-lanceolate; margins with fine to coarse serrations, sometimes biserrate. Mature female inflorescence somewhat compact (flowering stems producing small to medium “buds”), with relatively obscure sugar leaves (a high perigonal bract-to-leaf index); sugar leaves with capitate-stalked glandular trichomes (CSGTs) usually limited to the proximal half of the leaves; perigonal bracts express a moderate to high density of CSGTs. Mature achene exocarp color (beneath the perianth) often green-brown. | |
| A | THC/CBD ratio always ≥7, often much more. Mature achenes usually ≥ 3.6 mm long (Fig. 3e, f); perianth mostly sloughed off, but often persistent in places (appearing as irregular spots or stripes); exposed exocarp exhibiting prominent venation; lacking a prominent protuberant base; not readily disarticulating from plant | var. indica (“Sativa” in the historical sense2) |
| B | THC/CBD ratio usually ≥7, sometimes less. Mature achenes usually <3.6 mm long (Fig. 3g, h); perianth persistent (covering exocarp and its venation), with strong pigmentation in a mottled or striped pattern; with a protuberant base; readily disarticulating from plant | var. himalayensis |
Then there is what we are after here in this thread the wild and the domesticate
| 2. | Plants with a THC/CBD ratio <7; terpenoid profile includes sesquiterpene alcohols, fresh aroma often acrid or “skunky.” Plants < 2 m tall in good habitats, and often ca. 1 m; branches not flexible, branching sometimes nearly 90° from the stalk axis, producing a menorah-shaped habitus. Fresh leaves dark green in color, leaflets of larger leaves sometimes overlap; central leaflets broad (length/width usually <6), often oblanceolate; margins with coarse serrations, rarely biserrate. Mature female inflorescence compact (flowering stems producing medium to large “buds”) with prominent sugar leaves (a low perigonal bract-to-leaf index); sugar leaves have CSGTs extending more than half way down their length; perigonal bracts densely covered with CSGTs. Mature achene exocarp color (beneath the perianth) often a lighter shade of olive green to gray. | |
| A | THC/CBD ratio <7 (almost always >2). Mature achenes usually ≥ 3.6 mm long (Fig. 3a, b); perianth mostly sloughed off (appearing as irregular spots or stripes); exposed exocarp exhibiting prominent venation; lacking a prominent protuberant base; not disarticulating from plant, and often trapped in the dense inflorescence | var. afghanica (“Indica” in the historical sense2) |
| B | THC/CBD ratio often <2. Mature achenes usually < 3.6 mm long (Fig. 3c, d); perianth persistent (covering exocarp and its venation), with strong pigmentation in a mottled or striped pattern; with a protuberant base; readily disarticulating from plant | var. asperrima |
Sesquiterpenes are C15-terpenoids built from three isoprene units. They are found particularly in higher plants and in many other living systems such as marine organisms and fungi. Naturally, they occur as hydrocarbons or in oxygenated forms including lactones, alcohols, acids, aldehydes, and ketones.
Liktor, Erika & Keresztes, Attila & LaVigne, Justin & Streicher, John & Largent-Milnes, Tally. (2021). Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacological Reviews. 73. 98-126. 10.1124/pharmrev.120.000046.
Farnesyl pyrophosphate - Wikipedia
Last edited:
acespicoli
Well-known member
There is a referene to "Trinity" in the cannabible
TRINITY
These are easily some of the most pungent nuggets I have ever
come across. The overwhelming Skunk odor is so strong that even a
sealed jar won’t contain it. If you get pulled over with this stuff in
your car, you might as well just hand it over to the cop, because
there will be no denying what’s in the car! (“No, honestly, officer, I
just accidentally ran over five hundred skunks at once!”) This herb
was scored in Eugene, Oregon, where it commanded a higher price
than just about any other herb around. If that weren’t enough, this
particularly greedy grower sells it soaking wet! The desperate
connoisseurs still buy it all up, sending a message to the grower
that it is okay to sell this herb wet and overpriced. I do not support
these actions. Shame on him! As far as genetics go, I have been
informed that the Trinity is a three-way cross of select West Coast
genetics. This bud produces a knockout buzz and leaves a skunky
taste on the palate for hours.



Three-way hybrid of Northern California strains. The Trinity was bred in Wichita, Kansas and imported to Montana by Jeremy P. He then shared it with friends in Oregon. I have one of the last original mother plants of the Trinity which I have nurtured and shared with other local Caregivers. All things good come from the Trinity.
Medicinal applications include: pain relief, insomnia, and hyperactivity.
(info from bozemanbuds.com)
of course this is a selected clone from a reg seed line ommercially available in the 80s 90s maybe earlier
More Coming ...
Trinity (as described by Jason King in The Cannabible Vol. #1)
I'm looking for any information pertaining to this strain, be it mythical in origin or factual. I live in Oregon, Eugene to be precise and there is a particular pheno I'm interested in that used to be mass produced somewhere in this area. In the late 90's and early 00's we used to get a lot of...
www.icmag.com
TRINITY
These are easily some of the most pungent nuggets I have ever
come across. The overwhelming Skunk odor is so strong that even a
sealed jar won’t contain it. If you get pulled over with this stuff in
your car, you might as well just hand it over to the cop, because
there will be no denying what’s in the car! (“No, honestly, officer, I
just accidentally ran over five hundred skunks at once!”) This herb
was scored in Eugene, Oregon, where it commanded a higher price
than just about any other herb around. If that weren’t enough, this
particularly greedy grower sells it soaking wet! The desperate
connoisseurs still buy it all up, sending a message to the grower
that it is okay to sell this herb wet and overpriced. I do not support
these actions. Shame on him! As far as genetics go, I have been
informed that the Trinity is a three-way cross of select West Coast
genetics. This bud produces a knockout buzz and leaves a skunky
taste on the palate for hours.
Clone Only Strains Description
Variety: 75% Sativa, 25% IndicaThree-way hybrid of Northern California strains. The Trinity was bred in Wichita, Kansas and imported to Montana by Jeremy P. He then shared it with friends in Oregon. I have one of the last original mother plants of the Trinity which I have nurtured and shared with other local Caregivers. All things good come from the Trinity.
Medicinal applications include: pain relief, insomnia, and hyperactivity.
(info from bozemanbuds.com)
of course this is a selected clone from a reg seed line ommercially available in the 80s 90s maybe earlier
More Coming ...
acespicoli
Well-known member
Eugene, Oregon often comes up as a source of especially skunky afghani genetics
One cultivar that comes to mind is the berry selection "Blueberry"
off topic? maybe I have found both phenos skunky and berry
I have found both phenos skunky and berry
One cultivar that comes to mind is the berry selection "Blueberry"
Vintage Blueberry
An Alaskan native strain predating work by DJ Short that is highly rot and mildew resistant and is superb for harsh outdoor climates. Indoors, the plant needs a large pot to stretch out its roots as a constricted base will stress it into an autoflowering state that will show sex. Clean, upbeat, and happy.off topic? maybe
 I have found both phenos skunky and berry
I have found both phenos skunky and berryacespicoli
Well-known member
Functions of specialized terpenes. Defense against biological enemies is the best established function for plant terpenoids – whether directly through the targeting of herbivores as toxins or repellents, or indirectly through the attraction of predators or parasitoid enemies of such herbivores (Kessler & Heil, 2011).
Im just gonna leave this here...When in Jaguar country do not wear Obsession Cologne

 en.wikipedia.org
en.wikipedia.org
Functional Ecology Ó 2010 British Ecological SocietyFunctional Ecology 2011, 25, 348–357
Some plants such as Angelica archangelica or Abelmoschus moschatus produce musky-smelling macrocyclic lactone compounds. These compounds are widely used in perfumery as substitutes for animal musk or to alter the smell of a mixture of other musks.
The plant sources include the musk flower (Mimulus moschatus) of western North America, the muskwood (Olearia argophylla) of Australia, and the musk seeds (Abelmoschus moschatus) from India.
Im just gonna leave this here...When in Jaguar country do not wear Obsession Cologne

Civetone - Wikipedia
Functional Ecology Ó 2010 British Ecological SocietyFunctional Ecology 2011, 25, 348–357
Plants
Wikisource has the text of the 1905 New International Encyclopedia article "Musk Plant".Some plants such as Angelica archangelica or Abelmoschus moschatus produce musky-smelling macrocyclic lactone compounds. These compounds are widely used in perfumery as substitutes for animal musk or to alter the smell of a mixture of other musks.
The plant sources include the musk flower (Mimulus moschatus) of western North America, the muskwood (Olearia argophylla) of Australia, and the musk seeds (Abelmoschus moschatus) from India.
Last edited:
acespicoli
Well-known member
Loss of scent
Erythranthe moschata was widely grown and sold commercially in Victorian times for its fragrance, and is well known for the story that all cultivated and known wild specimens simultaneously lost their previous strong musk scent around the year 1913.[8] Writing in 1934 in the journal Nature, E. Hardy described a Lancashire nurseryman, Thomas Wilkinson, who in 1898 found that his plants developed a "rank, leafy smell"; five years later, after leaving the trade, he noticed that plants then on sale were scentless.[9] While it was sometimes claimed that strongly scented plants could still be found in the wild, Arthur William Hill, in a 1930 article in The Gardeners' Chronicle, presented evidence from British Columbia claiming that wild populations had also lost their scent.A variety of suggestions were put forward for a solution to the mystery, such as that the scent of the original cultivated form had been a rare recessive feature, which later disappeared as a result of uncontrolled pollination or the introduction of other genes from the wild population. Other explanations were given based on changes in climate, that humans had lost the ability to detect the smell, that the scent had been produced by a parasite, or that the loss of scent was a myth. W.B. Gourlay (1947) suggested that the phenomenon could be explained if the highly scented cultivated form had been reproduced vegetatively from a single aberrant plant, first raised in England from the original batch of seed, and later replaced by unscented plants grown from other sources of seed.[10] David Douglas first described the species and in 1826 near Fort Vancouver collected seed from which the first examples in England were raised; it is notable that he made no reference to a strong musk scent in his field notes.[11] Moreover, there are references as early as 1917 to plants in the wild having a wide range of characteristics between scentless and strongly scented, with the latter being "very much the exception".[12] During the 1920s and 1930s, at the height of botanists' interest in the 'lost scent' phenomenon, there were several reports of strongly-scented moschatus specimens being discovered in the wild, such as in 1931 on Texada Island, British Columbia

Erythranthe moschata - Wikipedia
Texada timewarp
Hello canadian growers, I'm wondering if the texada timewarp strain still exists? It seems it fueled the BC outdoor scene for many years. Can anyone point me in the direction of someone reputable for legit beanz
www.icmag.com
Last edited:
acespicoli
Well-known member
Plants
Wikisource has the text of the 1905 New International Encyclopedia article "Musk Plant".Some plants such as Angelica archangelica or Abelmoschus moschatus produce musky-smelling macrocyclic lactone compounds. These compounds are widely used in perfumery as substitutes for animal musk or to alter the smell of a mixture of other musks.
The plant sources include the musk flower (Mimulus moschatus) of western North America, the muskwood (Olearia argophylla) of Australia, and the musk seeds (Abelmoschus moschatus) from India.
This is the mimicry by a palatable species of an unpalatable or noxious species (the model), gaining a selective advantage as predators avoid the model and therefore also the mimic.
If the musky smell simulates the lion it repels the plant eating deer etc...?
acespicoli
Well-known member
Disgusting Food Museum - 80 of the world’s most disgusting foods.
Disgusting Food Museum invites visitors to explore the world of food and challenge their notions of what is and what isn’t edible. Could changing our ideas of disgust help us embrace the sustainable foods of the future?

Musk stick - Wikipedia
EXHIBITED DELICACIES INCLUDE:
- Surströmming – fermented herring from Sweden.
- Cuy – roasted guinea pigs from Peru.
- Casu marzu – maggot-infested cheese from Sardinia
- Stinky tofu – pungent bean curd from China.
- Hákarl – well-aged shark from Iceland.
- Durian – infamously stinky fruit from Thailand.

Durian - Wikipedia
Distribution and habitat
The large Indian civet ranges from Nepal, northeast India, Bhutan, Bangladesh to Myanmar, Thailand, the Malay Peninsula and Singapore to Cambodia, Laos, Vietnam and China.[1]In Nepal, the large Indian civet was recorded up to 2,250 m (7,380 ft) in the Himalayas.[6]
In China, the wild large Indian civet population declined drastically by 94–99% since the 1950s following deforestation, due to hunting for the fur trade, use of its musk glands as medicine and for the perfume industry.[3] By the 1990s, it was largely confined to the north of Guangdong Province in southern China, but has not been recorded in Hainan Island during surveys between 1998 and 2008.[7]

Large Indian civet - Wikipedia
Last edited:
Mithridate
Well-known member
Don't forget garum! Fermented fish gut sauce. You have to use the guts for their flora.
"Pliny the Elder was one of the first to define garum — which he called an “exquisite liquid” — as “a choice liquor consisting of the guts of fish and the other parts that would otherwise be considered refuse.”
"Pliny the Elder was one of the first to define garum — which he called an “exquisite liquid” — as “a choice liquor consisting of the guts of fish and the other parts that would otherwise be considered refuse.”







