It's this bit that I found a bit misleading. It sounds as if the double helix is formed by the DNA from mom and pop forming a double helix structure between themselves. Which is of course not the case.
Two DNA Molecule
DNA is a very long molecule that consists of different building blocks. The order of the building blocks forms a certain code a cell takes instructions from to produce certain substances and start processes.
Two DNA molecules attach to each other and intertwine like an old-fashioned phone cord. DNA and certain proteins form the chromosomes.
-
As of today ICMag has his own Discord server. In this Discord server you can chat, talk with eachother, listen to music, share stories and pictures...and much more. Join now and let's grow together! Join ICMag Discord here! More details in this thread here: here.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Sire Lines & "Y" They Matter
- Thread starter acespicoli
- Start date
acespicoli
Well-known member

Retrovirus - Wikipedia
- RNA: consists of a dimer RNA. It has a cap at the 5' end and a poly(A) tail at the 3' end. Genomic RNA (gRNA) is produced as a result of host RNA polymerase II (Pol II) activity and by adding a 5' methyl cap and a 3' poly-A tail is processed as a host mRNA.[10] The RNA genome also has terminal noncoding regions, which are important in replication, and internal regions that encode virion proteins for gene expression. The 5' end includes four regions, which are R, U5, PBS, and L. The R region is a short repeated sequence at each end of the genome used during the reverse transcription to ensure correct end-to-end transfer in the growing chain. U5, on the other hand, is a short unique sequence between R and PBS. PBS (primer binding site) consists of 18 bases complementary to 3' end of tRNA primer. L region is an untranslated leader region that gives the signal for packaging of the genome RNA. The 3' end includes three regions, which are PPT (polypurine tract), U3, and R. The PPT is a primer for plus-strand DNA synthesis during reverse transcription. U3 is a sequence between PPT and R, which serves as a signal that the provirus can use in transcription. R is the terminal repeated sequence at 3' end.
the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backward).
To me I need to study on this, the virus that got cannabis high goes back to basal cannabis
Very primitive sometimes herm lines due to evolution of cannabis reproduction to dioecy
The effects of the old sativas remember a few that were extreme


Retrotransposon - Wikipedia
Through reverse transcription, retrotransposons amplify themselves quickly to become abundant in eukaryotic genomes such as maize (49–78%)[3] and humans (42%).[4] They are only present in eukaryotes but share features with retroviruses such as HIV, for example, discontinuous reverse transcriptase-mediated extrachromosomal recombination.[5][6]
Last edited:
This may apply to fungi, I don't know, but it's not cannabis.I love that you came by and read that and let me know, let me see if I can get it right. Editing .... #28
.
In botany
. In land plants, by contrast, one generation – the sporophyte generation – consists of individuals that produce haploid spores rather than haploid gametes. Spores do not fuse, but germinate by dividing repeatedly by mitosis to give rise to haploid multicellular individuals, the gametophytes, which produce gametes. A male gamete and a female gamete then fuse to produce a new diploid sporophyte.[8]
acespicoli
Well-known member

Sex chromosome - Wikipedia
The interesting thing to me is the building blocks of life and phylogenic treesThis may apply to fungi, I don't know, but it's not cannabis.
I was thinking a single grain of pollen and a single ovule
Now break down that single grain of pollen whats in there ?
Fungus breeds very much like plants in my mind anyway...
monokaryotic mycelia dikaryotic
But im being fuzzy and lacking details maybe off thread topic at this point ?
You remeber my lacking a male and you had suggesting resurrecting my fem line the autosomes would work themselves out ?
high-throughput segregation analysis identifies the sex chromosomes of Cannabis sativa
- July 2019
Figure 3. Microsporogenesis and microgametogenesis developed in C. sativa. The different developmental stages are described as follows: (A–E) C. sativa var. Finola (dioecious male) staminate flowers growing in size. (F) C. sativa male flower after anthesis. (A′,A″) Tetrad stage. (B′,B″) Young and mid microspore stage. (C′,C″) Vacuolate microspore stage: arrows highlight apertures. (D′,D″) Young bi-cellular pollen stage. (E′,E″) Mid bi-cellular pollen stage. (F′,F″) Mature tri-cellular pollen stage. (A′–F′) Phase-contrast microscope images. (A″–F″) Fluorescent microscope images after DAPI staining. Scale bars (A–F): 1 mm; Scale bars (A′–F′) and (A″–F″): 10 μm. n, nucleus; v, vacuole; vg, vegetative nucleus; gn, generative nucleus; an, anther; fi, filament; se, sepal; s, spermatids.

Frontiers | Microgametophyte Development in Cannabis sativa L. and First Androgenesis Induction Through Microspore Embryogenesis
Development of double haploids is an elusive current breeding objective in Cannabis sativa L. We have studied the whole process of anther and pollen grain fo...

Spermatid - Wikipedia
-
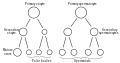
Scheme showing analogies in the process of maturation of the ovum and the development of the Genyo spermatids (young spermatozoa)
Last edited:
acespicoli
Well-known member
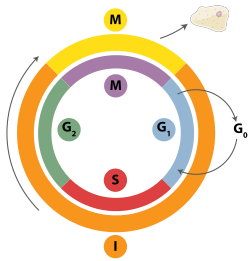
Figure 3.
Homologous recombination repair attempts occur in DNA
before the cell enters mitosis (M phase shown) during the S and G2 phases of the cell cycle.
In addition to repairing DNA, homologous recombination also helps produce genetic diversity when cells divide in meiosis to become specialized gamete cells—sperm or egg cells in animals, pollen or ovules in plants, and spores in fungi. It does so by facilitating chromosomal crossover, in which regions of similar but not identical DNA are exchanged between homologous chromosomes.[22][23] This creates new, possibly beneficial combinations of genes, which can give offspring an evolutionary advantage.[24]
The Secret History Of Sinsemilla
“OK, why don’t you grow a ton of seedless and bring it back to the States? We spent seven months with these two tribes, learning how to recognize and kill the male plants. Why don’t you bring a meaningful amount back to the States and fuck with the main chakra up there? Rattle some kundalini lines, which are dormant anyway.”It took two years to get it together. First, begging Indians to sell him handfuls of seeds. Then, going down the hill to search for grass traffickers with integrity. Planting a field and trying to explain why he would come back and destroy half the plants. Dealing with a semantic crisis:
Killing the machos was a negative symbol to Mexicans.
You don’t kill the males—who’s gonna fight?
Last edited:
acespicoli
Well-known member
4.1 | CSP-1 sex assayThe CSP-1 assay was found to be a reliable predictor of plantsex. There was a 50:50 segregation ratio in nearly all tested dioecious populations including CBD types, grain types, and grain/fiber types. As expected, monoecious plants were scored as fe-male (Divashuk et al., 2014). Given these data, it is likely that the CSP-1 assay distinguishes a nonrecombining part of the Ychromosome. This is somewhat surprising, given that the origi-nal MADC6 marker assay was not a completely accurate predic-tor of plant sex, with 2/75 reported recombinants (Törjék et al.,2002). It is possible that this was due to PCR failure, monoeciousplants with a quantitatively male phenotype, or that the CSP-1assay in fact examines a different DNA sequence than the origi-nal MADC6 assay. Recent C. sativa whole-genome sequencing(Laverty et al., 2019) showed six unassembled scaffolds in the male genome with >99% identity to the MADC6 sequence inC. sativa, possibly contributing to the empirical success of this assay. As MADC6 shows some sequence relationship to retro-transposons, it is possible that the sequence was subject to copy number increase in the recent past (Sakamoto, Ohmido, Fukui,Kamada, & Satoh, 2000; Törjék et al., 2002). It is well known that in the development of sex-determining regions of plants, an absence of recombination between male- and female-specific sequences can lead to an expansion of retrotransposon copy number repeats, which are not lost through a Muller's ratchet-type mechanism (Sakamoto et al., 2000; Vyskot & Hobza, 2004).
Last edited:
acespicoli
Well-known member
Mar 22, 2021
Volume 31Issue 6p1129-1352, R267-R310The rapid dissolution of dioecy by experimental evolution
Author links open overlay panelGuillaume G. Cossard 1, Jörn F. Gerchen 1, Xinji Li 1, Yves Cuenot 1, John R. Pannell 1 2
Show more
Add to Mendeley
Share
Cite
https://doi.org/10.1016/j.cub.2020.12.028
- •
Females without male mates evolved to produce increasing numbers of male flowers - •
Evolved females continued to sire progeny in competition with re-introduced males - •
Evolved females enjoyed reproductive assurance and an ability to outcross - •
Observed rates of evolution in the experiment were among the highest yet recorded
Evolutionary transitions from hermaphroditism to dioecy have been common in flowering plants,
1,2 but recent analysis also points to frequent reversions from dioecy to hermaphroditism.
2, 3, 4 Here, we use experimental evolution to expose a mechanism for such reversions, validating an explanation for the scattered phylogenetic distribution of dioecy.
We removed males from dioecious populations of the wind-pollinated plant Mercurialis annua and allowed natural selection to act on the remaining females that occasionally produced male flowers; such “leaky” sex expression is common in both males and females of dioecious plants.5
Over the course of four generations, females evolved a 23-fold increase in average male flower production. This phenotypic masculinization of females coincided with the evolution of partial self-fertilization, high average seed set in the continued absence of males, and a capacity to sire progeny when males were re-introduced into their populations.
Our study thus validates a mechanism for the rapid dissolution of dioecy and the evolution of functional hermaphroditism under conditions that may frequently occur during periods of low population density, repeated colonization, or range expansion.
6,7 Our results illustrate the power of natural selection, acting in replicated experimental populations, to bring about transitions in the mating behavior of plants.
Last edited:
acespicoli
Well-known member
Our recapitulation of the evolution of female flowers from
hermaphrodites, a step in the evolution of dioecy, was conducted
using the insect-pollinated species T. thalictroides. This scenario
assumes dioecy evolving before wind pollination, a sequence that
has been reported across angiosperms(Friedman and Barrett,
2008).

Link
Androdioecy can evolve either from hermaphroditic ancestors through the invasion of males or from dioecious ancestors through the invasion of hermaphrodites. The ancestral state is important because conditions under which androdioecy can evolve differ significantly.[citation needed]
 en.wikipedia.org
en.wikipedia.org


Link
hermaphrodites, a step in the evolution of dioecy, was conducted
using the insect-pollinated species T. thalictroides. This scenario
assumes dioecy evolving before wind pollination, a sequence that
has been reported across angiosperms(Friedman and Barrett,
2008).
Link
Evolution of androdioecy
The fitness requirements for androdioecy to arise and sustain itself are theoretically so improbable that it was long considered that such systems do not exist.[5][6] Particularly, males and hermaphrodites have to have the same fitness, in other words produce the same number of offspring, in order to be maintained. However, males only have offspring by fertilizing eggs or ovules of hermaphrodites, while hermaphrodites have offspring both through fertilizing eggs or ovules of other hermaphrodites and their own ovules. This means that all else being equal, males have to fertilize twice as many eggs or ovules as hermaphrodites to make up for the lack of female reproduction.[7][8]Androdioecy can evolve either from hermaphroditic ancestors through the invasion of males or from dioecious ancestors through the invasion of hermaphrodites. The ancestral state is important because conditions under which androdioecy can evolve differ significantly.[citation needed]
Androdioecy - Wikipedia
Link
Cannabis in Asia: its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies
Last edited:
acespicoli
Well-known member
Males are they even needed?
Characterization; Genome Sizes and Morphology of Sex Chromosomes in Hemp (Cannabis sativa L.) Koichi Sakamoto, Yukio Akiyama, Kiichi Fukui, Hiroshi Kamada and Shinobu Satoh https://www.jstage.jst.go.jp/article/cytologia1929/63/4/63_4_459/_pdf/-char/en the diploid genome sizes in female and...
www.icmag.com
Remembering the Genetics and Breeding conversations from past threads we discussed
It ultimately led to the creation of this thread, the questions we have as breeders.
I enjoyed the reversed pictures you shared in your thread, thanks for posting those
Hope this post finds you well

acespicoli
Well-known member
The five stages of sex chromosome evolution based on the size of the nonrecombining region, degree of degeneration, and size of Y chromosome.
Stage 1: Suppression of recombination at the sex determination locus and its neighboring regions led to mild degeneration of the suppressed region. YY genotype is viable.
Stage 2: Suppression of recombination continues to spread, and a small MSY region evolved. YY genotype is not viable.
Stage 3: The MSY expands in size and degenerates in gene content by accumulation of transposable element insertions and intrachromosomal rearrangements. The X and Y chromosomes become heteromorphic.
Stage 4: Severe degeneration of the Y chromosome causes loss of function for most genes. Deletion of nonfunctional DNA sequences results in shrinking of the Y chromosome in size.
Stage 5: Suppression of recombination spreads to the entire Y chromosome. The Y chromosome is lost, and X-to-autosome ratio sex determination system has evolved.
Last edited:
acespicoli
Well-known member
Sexchromosomes-AJB.pdf
 drive.google.com
drive.google.com
Sex chromosomes in dioecious and polygamous plants evolved as a mechanism for ensuring outcrossing to increase genetic variation in the offspring. Sex specificity has evolved in 75% of plant families by male sterile or female sterile mutations, but well-defined heteromorphic sex chromosomes are known in only four plant families. A pivotal event in sex chromosome evolution, suppression of recombination at the sex determination locus and its neighboring regions, might be lacking in most dioecious species. However, once recombination is suppressed around the sex determination region, an incipient Y chromosome starts to differentiate by accumulating deleterious mutations, transposable element insertions, chromosomal rearrangements, and selection for male-specific alleles. Some plant species have recently evolved homomorphic sex chromosomes near the inception of this evolutionary process, while a few other species have sufficiently diverged heteromorphic sex chromosomes. Comparative analysis of carefully selected plant species together with some fish species promises new insights into the origins of sex chromosomes and the selective forces driving their evolution.
acespicoli
Well-known member
Theor Appl Genet (2003) 107:102–109 DOI 10.1007/s00122-003-1212-5 A. Peil · H. Flachowsky · E. Schumann · W. E. Weber Sex-linked AFLP markers indicate a pseudoautosomal region in hemp (Cannabis sativa L.) Received: 30 April 2002 / Accepted: 18 November 2002 / Published online: 20 February 2003 Springer-Verlag 2003 Abstract In dioecious plants of hemp (Cannabis sativa L.), males are regarded as heterogametic XY and females as homogametic XX, although it is difficult to discriminate the X cytologically from the Y. The Y chromosome is somewhat larger than the X. Our aim was to analyse AFLP markers on X and Y, and to use them to gain some insight into the structure of the sex chromosomes. Markers located on the sex chromosomes can be grouped into different classes, depending on the presence or absence of a fragment on the X and/or the Y. They are detected by separately analysing male and female progenies of a single cross. Five markers were found to be located on both chromosomes. A few recombinants were observed for marker pairs of this class in the male progenies. Two completely linked markers located on the Y chromosome in the male parent show a recombination rate of r = 0.25 with sex. Recombination must have occurred between the sex chromosomes in the male parent. The recombination analysis led to the conclusion that there is a pseudoautosomal region (PAR) on the sex chromosomes, allowing recombination between the X and the Y chromosome. The other regions of the sex chromosomes show only a few recombination events, for the Y as well as for the X. These results are discussed in comparison to other dioecious plants. Keywords Hemp · Recombination · Pseudoautosomal region · Sex chromosomes · AFLP
Sex-linked_AFLP_markers_indicate_a_pseudoautosomal.pdf
 drive.google.com
drive.google.com
acespicoli
Well-known member
acespicoli
Well-known member
Figure 3. Three scenarios of transgenerational proliferation of transposable elements (TEs) in plants and their impact on the chromosomal distribution of a TE. Red dots indicate TE insertions—in blue-colored meiotic chromosomes, A-autosomes, X-chromosomes, and Y-chromosomes. Numbers next to double arrows indicate the expected density of TE insertions per one unit of length of a respective chromosome. (A) If a TE is passed down to offspring equally in males and females, TE insertion density is identical on all chromosomes. (B) If TE proliferation is disrupted in males, TE density is 1,33 times higher on the X chromosome than on autosomes, but nearly zero on the Y chromosome. (C) If TE proliferation is disrupted in females, TE insertion density is lower on the X chromosome compared to autosomes and twice as high on the Y chromosome than on autosomes. These three scenarios represent extreme cases of sex-specific TE activity. Real world TEs range from TEs that are almost fully sex-specifically inheritable to TEs with only slight sex-dependent inheritance.
6. Conclusions
Although the advent of genomic methods has shed light on many aspects of heteromorphic sex chromosome formation in dioecious plants, there is still limited information about the impact of structural changes on the function of sex-linked genes. Transposable elements can affect sex chromosome evolution directly via insertion into a specific site, and indirectly by affecting the expression of closely linked genes by epigenetic mechanisms. Large-scale genomic response to repetitive DNA accumulation results in changes in chromatin status, which can in some species lead to heterochromatinization. Is an elevated rate of transposon accumulation the cause or consequence of sex chromosome degeneration? How much does cross-talk of transposable elements with genic regions affect dosage compensation evolution? How much are epigenetic processes involved in the degeneration of sex chromosomes? Surprisingly, structurally divergent sex chromosomes in S. latifolia are euchromatic while papaya homomorphic sex chromosomes reveal clear signs of heterochromatization. It is likely that sex chromosome evolution is affected by a number of mechanisms that vary in individual dioecious species such as population size, genome dynamics, regulation of TEs, etc. It remains to be answered which processes are shared among the species and which mechanisms are unique in individual species. Recent studies clearly show that plants possessing sex chromosomes can regulate the activity of TEs and subsequently regulate their spread in non-recombining regions. Whether this phenomenon is specific for dioecious plants or it is a common attribute of angiosperms remains to be elucidated. It is tempting to speculate that not only RNAi (RNA interference) machinery but also specific DNA conformation such as quadruplexes may play a role in the dynamics of the spread of repetitive elements within sex chromosomes.Impact of Repetitive Elements on the Y Chromosome Formation in Plants
1
Department of Plant Developmental Genetics, Institute of Biophysics, Academy of Sciences of the Czech Republic, Kralovopolska 135, 61200 Brno, Czech Republic
2
Centre of the Region Hana for Biotechnological and Agricultural Research, Institute of Experimental Botany, 78371 Olomouc, Czech Republic
*
Author to whom correspondence should be addressed.
Genes 2017, 8(11), 302; https://doi.org/10.3390/genes8110302
Submission received: 21 August 2017 / Revised: 19 October 2017 / Accepted: 26 October 2017 / Published: 1 November 2017
acespicoli
Well-known member
Figure 1.
Chromosomes of C. sativa: C-banding/DAPI, male metaphase (a), male prometaphase (b) and female metaphase (c).Distribution of Arabidopsis-type (5′-CCCTAAA-3′) telomeric sequences (small red dots located at the end of the chromosomes) (d). FISH with 45S rDNA (green signals) on male (e) and female (f) metaphase chromosomes. Bicolor FISH to male metaphase (g) with CS-1 subtelomeric repeat (red) and 5S rDNA probe (green). FISH to female metaphase (h) with CS-1 subtelomeric repeat (red). The karyotypes of male (i) and female (j) plants. Bar = 5 µm.
More »
Figure 2.
Idiogram of haploid chromosome complement of C. sativa, including Arabidopsis-type telomeric repeat, CS-1 subtelomeric repeat, 45S rDNA and 5S rDNA sites.More »
Table 1.
Size of sex chromosomes compared to the largest autosome.More »
Figure 3.
The meiotic chromosomes of C. sativa at diakinesis (a) and metaphase I (b).The chiasma between the sex chromosomes can clearly be seen and indicated by arrows. c Idiogram of the C. sativa XY chromosomes with the hybridization sites of CS-1 (green) and the Arabidopsis-type telomeric repeat (red). The pseudoautosomal region is indicated by brackets.
More »
Figure 4.
Simplified phylogeny of Cannabaceae genus included in idiograms.The phylogeny is according to the reference of [59]. Idiograms created based on data obtained in [21], [23], [24], [26], [55] and in this study. 5S rDNA: green signals; 45S rDNA: red signals; species-specific subtelomeric repeats (HSR-1for H. lupulus, HJSR for H. japonicus and CS-1 for C. sativa): green signal. The position of pseudoautosomal region on sex chromosomes is indicated by brackets. Time of divergence estimated in [60], [61], [62].
More »
acespicoli
Well-known member
Results
ERGs are conserved in C. sativa
Based on 47 AtERGs previously identified (Supplementary Table 2), ortholog analyses identified 43 putative CsERGs (Table 1). At least one ortholog of each key gene in the canonical ethylene biosynthesis and signaling pathways, as well as the Yang cycle, was identified in silico except for auxin-regulated genes involved in organ size (AtARGOS; Supplementary Table 2). Analysis of the amino acid sequences of the CsERGs also confirmed the presence of most conserved domains found in the AtERGs (see Supplementary Table 3). To the best of our knowledge, this is the first time that the genes coding for the various enzymes involved in the putative Yang cycle (Figure 2a), ethylene biosynthesis (Figure 2b) and signaling pathway (Figure 3) have been proposed in C. sativa. These genes are available in a collection on the NCBI site at: https://www.ncbi.nlm.nih.gov/sites/myncbi/adrian.monthony.1/collections/63471644/public/.C. sativa model of the Yang cycle and ethylene biosynthesis determined by homology analysis from A. thaliana. Products of enzymatic reactions are italicized. Genes encoding enzymes are in boxes. (a) Yang cycle-product abbreviations are as follows: S- adenosylmethionine (SAM), 5-methylthioadenosine (MTA), 5-methylthioribose (MTR), 5-methyltioriobose-1-P (MTR-1-P), 5-methylthioribulose-1-P (MTRu-1-P), 1,2-dihydroxy-3-keto-5-methylthiopentene (DHKMP), 2-keto-4-methylthiobutyrate (KMTB), methionine (Met). (b) Ethylene biosynthesis-product abbreviations are as follows: 1-aminocyclopropane-1-carboxilic acid (ACC). Differentially expressed genes are annotated with Mars and Venus symbols, identifying their proposed roles as either involved in sexual determinism (sex determination; denoted with diverging arrows) or sexual plasticity (denoted with reciprocal arrows) based on RNA-seq analysis.
" data-icon-position="" data-hide-link-title="0" style="-webkit-font-smoothing: antialiased; margin: 0px; padding: 8px; border: none; outline: 0px; vertical-align: baseline; font-style: inherit; font-variant: inherit; font-weight: 400; font-stretch: inherit; font-size: 0.857rem; line-height: inherit; font-family: inherit; font-optical-sizing: inherit; font-kerning: inherit; font-feature-settings: inherit; font-variation-settings: inherit; text-decoration: none; color: gray; display: block; box-shadow: rgba(0, 0, 0, 0.15) 0px 2px 10px 0px; background: rgb(255, 255, 255);">

Figure 2
C. sativa model of the Yang cycle and ethylene biosynthesis determined by homology analysis from A. thaliana. Products of enzymatic reactions are italicized. Genes encoding enzymes are in boxes. (a) Yang cycle-product abbreviations are as follows: S- adenosylmethionine (SAM), 5-methylthioadenosine (MTA), 5-methylthioribose (MTR), 5-methyltioriobose-1-P (MTR-1-P), 5-methylthioribulose-1-P (MTRu-1-P), 1,2-dihydroxy-3-keto-5-methylthiopentene (DHKMP), 2-keto-4-methylthiobutyrate (KMTB), methionine (Met). (b) Ethylene biosynthesis-product abbreviations are as follows: 1-aminocyclopropane-1-carboxilic acid (ACC). Differentially expressed genes are annotated with Mars and Venus symbols, identifying their proposed roles as either involved in sexual determinism (sex determination; denoted with diverging arrows) or sexual plasticity (denoted with reciprocal arrows) based on RNA-seq analysis.
A simplified model of ethylene signaling in C. sativa, constructed through computational analysis using the A. thaliana pathway as a template. Pathway is depicted in the presence (a) and absence-(b) of ethylene. Dashed lines represent genes not found in C. sativa. Pointed arrows indicate promotion, while blunt-end arrows represent repression. The figure design is based on existing ethylene signaling models (Binder et al., 2010; Dubois et al., 2018; Binder, 2020). Differentially expressed genes are marked with Mars and Venus symbols, indicating their proposed roles in sexual determinism (sex determination, diverging arrows) or sexual plasticity (reciprocal arrows) based on RNA-seq analysis.
" data-icon-position="" data-hide-link-title="0" style="-webkit-font-smoothing: antialiased; margin: 0px; padding: 8px; border: none; outline: 0px; vertical-align: baseline; font-style: inherit; font-variant: inherit; font-weight: 400; font-stretch: inherit; font-size: 0.857rem; line-height: inherit; font-family: inherit; font-optical-sizing: inherit; font-kerning: inherit; font-feature-settings: inherit; font-variation-settings: inherit; text-decoration: none; color: gray; display: block; box-shadow: rgba(0, 0, 0, 0.15) 0px 2px 10px 0px; background: rgb(255, 255, 255);">

Figure 3
A simplified model of ethylene signaling in C. sativa, constructed through computational analysis using the A. thaliana pathway as a template. Pathway is depicted in the presence (a) and absence-(b) of ethylene. Dashed lines represent genes not found in C. sativa. Pointed arrows indicate promotion, while blunt-end arrows represent repression. The figure design is based on existing ethylene signaling models (Binder et al., 2010; Dubois et al., 2018; Binder, 2020). Differentially expressed genes are marked with Mars and Venus symbols, indicating their proposed roles in sexual determinism (sex determination, diverging arrows) or sexual plasticity (reciprocal arrows) based on RNA-seq analysis.
Position of CsERGs in the cs10 reference genome identified from A. thaliana orthologs. (a) Distribution of CsERGs across the genome. Distinct colors were used to describe their functional annotation. (b) Proportion of CsERGs distributed on autosomes or ChrX. An interactive version of (a) can be found in the on the OSF page for this project.
" data-icon-position="" data-hide-link-title="0" style="-webkit-font-smoothing: antialiased; margin: 0px; padding: 8px; border: none; outline: 0px; vertical-align: baseline; font-style: inherit; font-variant: inherit; font-weight: 400; font-stretch: inherit; font-size: 0.857rem; line-height: inherit; font-family: inherit; font-optical-sizing: inherit; font-kerning: inherit; font-feature-settings: inherit; font-variation-settings: inherit; text-decoration: none; color: gray; display: block; box-shadow: rgba(0, 0, 0, 0.15) 0px 2px 10px 0px; background: rgb(255, 255, 255);">

Figure 4
Position of CsERGs in the cs10 reference genome identified from A. thaliana orthologs. (a) Distribution of CsERGs across the genome. Distinct colors were used to describe their functional annotation. (b) Proportion of CsERGs distributed on autosomes or ChrX. An interactive version of (a) can be found in the on the OSF page for this project.
Table 1Cannabis sativaethylene-related genes (CsERGs) identified through ortholog analysis.
Differential expression analysis reveals three patterns of expression
Among the 43 putative CsERGs analyzed, a total of 35 were found to be expressed in both dataset 1 and dataset 2, as indicated in Table 1. Upon further investigation, we found that among these 35 CsERGs, 16 exhibited differential expression between flower types in dataset 1, while 11 showed differential expression in dataset 2, as shown in Supplemental Figure 2. Seven CsERGs that consistently displayed differential expression between MFs and FFs were identified across both datasets, with comparable levels of magnitude, as illustrated in Supplemental Figure 3. These 7 CsERGs are considered high confidence genes involved in cannabis sex determination or sexual plasticity. In conducting pairwise comparisons of CsERG expression between different flower types, we observed three distinct patterns of differential expression. These patterns are described below and represent a newly proposed framework for understanding the role of CsERGs in sex determination and sexual plasticity. These patterns have been classified as karyotype concordant (KC-sex determination; Figure 5), floral organ concordant (FOC-sexual plasticity; Figure 6), and unique ERG (uERG-sexual plasticity; Figure 7).Differential expression analysis of C. sativa ERGs revealed nine genes with a karyotype concordant (KC) expression pattern. Among these genes, female flowers (FF; XX karyotype) and induced male flowers (IMF; XX karyotype) showed not significant (ns) differential expression. Significant pairwise comparisons are denoted by asterisks: ns = not significant; * for p ≤ 0.05; ** for p ≤ 0.01; *** for ≤ 0.001 as determined by the Wald test.
" data-icon-position="" data-hide-link-title="0" style="-webkit-font-smoothing: antialiased; margin: 0px; padding: 8px; border: none; outline: 0px; vertical-align: baseline; font-style: inherit; font-variant: inherit; font-weight: 400; font-stretch: inherit; font-size: 0.857rem; line-height: inherit; font-family: inherit; font-optical-sizing: inherit; font-kerning: inherit; font-feature-settings: inherit; font-variation-settings: inherit; text-decoration: none; color: gray; display: block; box-shadow: rgba(0, 0, 0, 0.15) 0px 2px 10px 0px; background: rgb(255, 255, 255);">

Figure 5
Differential expression analysis of C. sativa ERGs revealed nine genes with a karyotype concordant (KC) expression pattern. Among these genes, female flowers (FF; XX karyotype) and induced male flowers (IMF; XX karyotype) showed not significant (ns) differential expression. Significant pairwise comparisons are denoted by asterisks: ns = not significant; * for p ≤ 0.05; ** for p ≤ 0.01; *** for ≤ 0.001 as determined by the Wald test.
Differential expression analysis of C. sativa ERGs revealed five cases of genes showing floralorgan concordant (FOC) expression. FOC refer to genes whose expression pattern is shared between organisms with the same floral organ, but not necessarily the same sex-chromosome karyotype designated by ns in transcription levels for male flowers on XY plants and induced male flowers on XX plants. S0 Significant pairwise comparisons are denoted by asterisks: ns = not significant; * for p ≤ 0.05; ** for p ≤ 0.01; *** for ≤ 0.001 as determined by the Wald test.
" data-icon-position="" data-hide-link-title="0" style="-webkit-font-smoothing: antialiased; margin: 0px; padding: 8px; border: none; outline: 0px; vertical-align: baseline; font-style: inherit; font-variant: inherit; font-weight: 400; font-stretch: inherit; font-size: 0.857rem; line-height: inherit; font-family: inherit; font-optical-sizing: inherit; font-kerning: inherit; font-feature-settings: inherit; font-variation-settings: inherit; text-decoration: none; color: gray; display: block; box-shadow: rgba(0, 0, 0, 0.15) 0px 2px 10px 0px; background: rgb(255, 255, 255);">

Figure 6
Differential expression analysis of C. sativa ERGs revealed five cases of genes showing floralorgan concordant (FOC) expression. FOC refer to genes whose expression pattern is shared between organisms with the same floral organ, but not necessarily the same sex-chromosome karyotype designated by ns in transcription levels for male flowers on XY plants and induced male flowers on XX plants. S0 Significant pairwise comparisons are denoted by asterisks: ns = not significant; * for p ≤ 0.05; ** for p ≤ 0.01; *** for ≤ 0.001 as determined by the Wald test.
Differential expression analysis of revealed unique ERG (uERG) expression, which are cases where gene expression in the sex-changed plant does not match either that of the plant with which is shares the same sex chromosome karyotype or the plant with which is shares the same floral organs. Significant pairwise comparisons are denoted by asterisks: ns = not significant; * for p ≤ 0.05; ** for p ≤ 0.01; *** for ≤ 0.001 as determined by the Wald test.
" data-icon-position="" data-hide-link-title="0" style="-webkit-font-smoothing: antialiased; margin: 0px; padding: 8px; border: none; outline: 0px; vertical-align: baseline; font-style: inherit; font-variant: inherit; font-weight: 400; font-stretch: inherit; font-size: 0.857rem; line-height: inherit; font-family: inherit; font-optical-sizing: inherit; font-kerning: inherit; font-feature-settings: inherit; font-variation-settings: inherit; text-decoration: none; color: gray; display: block; box-shadow: rgba(0, 0, 0, 0.15) 0px 2px 10px 0px; background: rgb(255, 255, 255);">

Figure 7
Differential expression analysis of revealed unique ERG (uERG) expression, which are cases where gene expression in the sex-changed plant does not match either that of the plant with which is shares the same sex chromosome karyotype or the plant with which is shares the same floral organs. Significant pairwise comparisons are denoted by asterisks: ns = not significant; * for p ≤ 0.05; ** for p ≤ 0.01; *** for ≤ 0.001 as determined by the Wald test.
KC expression refers to CsERGs that displayed no significant differences in expression between plants with the same sex chromosome karyotype, such as FFs and IMFs. Among the 16 CsERGs exhibiting differential expression, 9 were identified to have KC expression, primarily in dataset 1, as depicted in Figure 5. Conversely, FOC expression describes genes with similar expression between MFs and IMFs, which possess the same floral organs, but that had significantly different expression when compared with FF. Out of the 16 differentially expressed CsERGs, five displayed FOC expression, as indicated in Figure 6. Notably, three genes (CsACS2, CsFYF2, and CsFYF4) exhibited higher expression in FFs compared to phenotypic male flowers, whereas two genes (CsETP2 and CsMTN) demonstrated higher expression in MFs and IMFs compared to FFs, as illustrated in Figure 6. Lastly, uERG expression referred to CsERGs that exhibited significant differential expression among each floral type, including FFs, MFs, and IMFs. Two CsERGs, namely CsACO5 and CsFYF1, were identified to possess uERG expression, as depicted in Figure 7. By proposing this framework, we aim to deepen our understanding of the involvement of CsERGs in sex determination and sexual plasticity in cannabis.
ERGs are distributed genome-wide in C. sativa
The 43 CsERGs identified in the homology analysis were distributed across the genome (Figure 4 and Supplementary Table 4), independently of autosomes or sex chromosomes. Approximately 74% (32) of the CsERGs were located on autosomes (Figure 4b). Yang cycle genes constituted the majority (40%; 3) of CsERGs on the X chromosome. In contrast, none of the genes that were classified as ethylene adjacent (ERGs from the literature not belonging to the canonical ethylene biosynthesis or signaling pathways) were found on the X chromosome (Figure 4). Among the 43 CsERGs identified, all but one (CsACO1-LIKE1) mapped to chromosomes in the reference genome, with CsACO1-LIKE1 located on an unassigned scaffold (Figure 4a). CsERGs exhibiting significant differential expression associated with sex determination (KC) and sexual plasticity (FOC and uERG) were distributed throughout the genome (Figure 4a), encompassing genes from the Yang Cycle and ethylene biosynthesis pathway (Figure 2) as well as the ethylene signaling pathway (Figure 3). Approximately 37% of the ERGs (16) displayed a pattern of differential expression, among which only three were mapped to the sex chromosomes (Figure 4a). Specifically, CsEIL1 exhibited KC expression patterns, while CsACO5 and CsMTN showed FOC/uERG expression. The remaining 13 differentially expressed genes were located on various autosomes (Figure 4a), except for chromosomes 4 and 6, which lacked any differentially expressed ERGs.Discussion
To date, sexual plasticity has only been discussed in the context of the animal kingdom (Liu et al., 2017). While studies have focused on sex determination in angiosperms without sex chromosomes, such as the Cucurbitaceae family (Yamasaki et al., 2001; Boualem et al., 2015; Oda et al., 2022), sexual plasticity in plants remains relatively unexplored. However, as a dioecious plant with sex chromosomes, cannabis represents an uncommon example of a plant species that exhibits sexual plasticity. Previous research has demonstrated the induction of male flowers in female plants through the inhibition of ethylene using STS (Lubell & Brand, 2018), and the induction of female flowers in male plants through ethylene (ethephon) treatment (Moon et al., 2020a). Considering ethylene’s known involvement in sex determination across plant species, it serves as a promising starting point for investigating sexual plasticity in dioecious plants like C. sativa (Hume & Lovell, 1983; Manzano et al., 2014; Cebrián et al., 2022). However, unraveling the specific mechanisms by which ethylene influences sexual plasticity presents challenges due to its multifaceted roles in plants.To understand the influence of CsERGs on sex determination and sexual plasticity, we propose a framework consisting of three putative CsERG expression patterns: karyotype concordant (KC; Figure 5), floral organ concordant (FOC; Figure 6), and unique ERG (uERG; Figure 7). KC expression is similar between individuals with the same sex chromosome karyotype and CsERGs exhibiting KC expression are unaffected by STS treatments that induce sexual plasticity in C. sativa. This type of expression suggests that genes belonging to the KC case may have sex-specific patterns of expression, playing a role in sex determination. Conversely, FOC expression (Figure 6) is the same between individuals with shared floral organ sex, but carrying different sex chromosome karyotypes. This pattern suggests that CsERGs showing FOC expression are involved in sexual plasticity, as their expression appears uncoupled from the sex chromosome karyotypes. uERGs (Figure 7) shows a pattern of distinct levels of expression of CsERGs in all three treatments. Two genes, CsFYF1 and CsACO5, showed uERG expression, with the gene expression in the sex-manipulated plant (IMF) not matching either of the plants with the same genotypic sex (FF) or the plant with the phenotypic sex (MF). Below, we discuss how each of these patterns is found throughout the biochemical journey of ethylene, from its precursor producing in the Yang cycle (Figure 2a) all the way through ethylene biosynthesis (Figure 2b) and signaling (Figure 3). We also highlight that the distribution of these differentially expressed CsERGs is genome-wide and not exclusive to the sex chromosomes (Figure 4). Lastly, we end by discussing limitations and future opportunities revealed by this study.
High expression of key Yang Cycles genes; a potential indicator of masculinity in C. sativa
In previous studies, it has been demonstrated that changes in the expression of Yang cycle genes regulate ethylene production in plants. Here, we explore the potential role of the Yang cycle in cannabis sex determination and sexual plasticity. In the present study, we found that two CsERGs from the Yang cycle showed KC expression (Figure 2a and Figure 5), methionine adenosyltransferase 3 (CsMAT3) and acireductone dioxygenase 2 (CsARD2). Most higher eukaryotes possess small ARD gene families (Sauter et al., 2005). A. thaliana has four ARD genes isoforms (Pommerrenig et al., 2011) from which we identified two CsARD genes homologs. In the dataset 1, CsARD2 was more highly expressed in MFs than FFs and IMFs (Figure 5). In contrast CsARD1 transcripts were found in all treatments, in both datasets, but expression did not vary significantly between MFs, FFs or IMFs. These findings are consistent with previous studies in rice that showed that the different ARD isoforms are regulated differently in planta, with OsARD1 undergoing a transient increase in expression in response to submergence, while OsARD2 is constitutively expressed, and unaffected by changes in ethylene concentration (Sauter et al., 2005). Similarly, Liang et al. (2019) showed that transgenic rice overexpressing OsARD1 produced higher levels of ethylene versus wild-type rice.Methionine adenosyltransferase (MAT, or SAM synthetase; SAMS) is responsible for the biosynthesis of SAM, an important methyl donor and precursor for ethylene, lignin and polyamine synthesis (Arraes et al., 2015). From the four AtMAT used to construct the putative Yang cycle model in cannabis, we identified three putative CsMAT genes: CsMAT1, CsMAT2, CsMAT3, of which CsMAT3 was found to have a KC expression. CsMAT3 was most expressed in MFs, with significantly lower transcription levels in FFs and IMFs. The association MAT genes with male floral characteristics have been reported in Arabidopsis by Chen et al. (2016b) who found that expression of AtMAT3, a CsMAT3 ortholog, is responsible for normal pollen germination and pollen tube formation. Although critical male fertility and pollen germination in Arabidopsis, the role of MAT genes in controlling sex determination has yet to be reported. The present findings that CsMAT3 gene expression is higher in MFs than in FFs, suggest that it may play a role in sex determination. One way that CsMAT3 may affect sex determination is by altering levels of DNA methylation. SAM, the product of MAT is a methyl donor for DNA and histone methylation, and it has been reported that mat4 knockouts in Arabidopsis have lower genome-wide methylation levels as well as decreased histone methylation (Meng et al., 2018). Similar findings have been echoed by Chen et al. (2016b) who reported that MAT3 was required for proper histone and tRNA methylation. It is possible that higher expression of CsMAT3 in male plants could lead to increased methylation of key genes involved in plant masculinity. To date, there has been a scarcity of research on the methylation patterns of cannabis (Aina et al., 2004; Mayer et al., 2015), with no studies investigating its role in sex determination and sexual plasticity. Investigating the methylation status of floral tissues in cannabis may yield valuable insights into the mechanisms underlying sex determination and sexual plasticity in this species.
Here, we report the identification of one CsERG from the Yang cycle, CsMTN, with a FOC expression. MTN recycles MTA after the first step of the ethylene biosynthesis cycle where SAM is converted to ACC by ACS. The ortholog analysis for both AtMTN1 and AtMTN2 revealed a single ortholog in cannabis (Table 1), which is similar to both rice (Oryza sativa L.) and tomato (Solanum lycopersicum L. Karst. ex Farw) in which MTN is only encoded by one gene (Rzewuski et al., 2007; van de Poel et al., 2012). In Arabidopsis, MTN shows preferential expression in the phloem (Pommerrenig et al., 2011), suggesting that tissue sampling may also play a role in the expression patterns of MTN. In dataset 1 CsMTN transcripts were most abundant in MFs (Supplemental Figure 3), and in dataset 2 (Figure 6) CsMTN transcripts were significantly more expressed in MFs and IMFs than in FFs, suggesting that high abundance of CsMTN may be associated with masculinity and sexual plasticity in cannabis.
Multiple biosynthesis genes linked to sexual plasticity
Ethylene biosynthesis is regulated by a set of conserved enzymes that are found in all plant species. The key enzyme in this two-step process is ACS, which converts SAM, derived from the Yang cycle, into ACC. ACC is then converted into ethylene by the enzyme ACO. These enzymes are conserved across all plant species, and their regulation is tightly controlled by various factors such as hormonal and environmental cues (Bleecker & Kende, 2000). The ortholog analysis found that ACS and ACO belong to multi-gene families in cannabis, similar to Arabidopsis. In C. sativa, 6 ACS and 5 ACO gene homologs were identified from the 8 ACS and 5 ACO genes in A. thaliana respectively (Figure 2 and Table 1). The presence of ACS and ACO are critical to ethylene biosynthesis, but the number of genes in each family can vary between species. For example, in Pyrus ussuriensis L. (pear) 13 ACS and 11 ACO genes have been identified (Yuan et al., 2020), in Zea mays L. 5 ACS and 15 ACO genes have been identified, in rice 6 ACS and 9 ACO genes, and in tomato there are 9 ACS and 7 ACO genes (Houben & Van de Poel, 2019; Wu et al., 2022). The importance of having multiple functionally redundant ethylene biosynthesis genes has been demonstrated in multiple plants, with studies showing ACS and ACO genes are expressed at different levels and in different tissues as a function of the plant organ’s needs and developmental stage (van de Poel et al., 2012; Van de Poel et al., 2014). In ripening tomatoes Van de Poel et al. (2012) reported a higher level of ACS6, ACO2 and ACO4 expression during baseline (system 1) ethylene production during immature fruit development prior to ripening, but that auto-catalytic increases in ethylene during ripening (system 2) were correlated with increases in ACS2, ACS4, ACO3 and ACO5 expression, while abundance of ACO2 and ACO4 transcripts decreased at that same stage. The induction/repression of specific ACS genes has also been shown to play a role in sex determination of some Cucurbitaceae species (Manzano et al., 2014; Boualem et al., 2015; Chen et al., 2016a; Zhang et al., 2017). One study found that expression of ACS11 was only observed in the vascular bundles of female flowers of monoecious and in hermaphroditic flowers of andromonoecious plants (plants producing male and hermaphrodite flowers), pointing to the role of ACS11 in carpel development (Boualem et al., 2015). Changes in sex determination have also been shown in cucumber and melon by way of disruption of a specific ACO genes (ACO2 in cucumber; ACO3 in melon) resulting in decreased ethylene production in the carpel region of flowers and causing a shift from a monoecy to androecy resulting in unisexual flower production in cucumber and melons (Chen et al., 2016a). The existence of multiple, functionally redundant ACS and ACO genes, combined with their spatial and temporal expression, allows for the fine tuning of ethylene biosynthesis. This gives plants a high level of control over their development, including sex determination. The present study demonstrates evidence of numerous ACS and ACO genes distributed throughout the genome of C. sativa (Table 1 and Figure 4). Notably, the expression patters of CsACS2 (Figure 6), CsACO3 (Figure 5) and CsACO5 (Figure 7) represent the three distinct cases of gene expression proposed in this study. These findings collectively suggest a diverse range of effects exerted by the ethylene biosynthesis pathway on cannabis sex determination and sexual plasticity.CsACS2, which is part of the ACS gene family responsible for the first step of ethylene biosynthesis, shows a FOC expression linking it to sexual plasticity. ACS has been demonstrated to catalyze the rate-limiting reaction in most cases of ethylene production (van de Poel et al., 2012; Houben & Van de Poel, 2019; Pattyn et al., 2021). This gene is downregulated in MFs and IMFs in comparison to FFs. Comparisons in CsACS2 expression show similar low expression levels between MFs and IMFs despite their different genotypic sex. Since ACS is thought to be rate-limiting, and given the association of ethylene with ‘feminization’ of floral phenotypes we hypothesize that the downregulation of CsACS2 in IMF relative to FF, points to reduction in ethylene biosynthesis in planta which may play a role in the induction of male flowers in genetically female plants. CsACO3 shows KC expression, with activity in MFs much higher for both than in IMF and FFs. Further study which incorporates multiple tissue sources could prove helpful in understanding CsACO3’s KC expression and how this plays a putative role in sex determination, as it remains to be seen if this expression pattern is unique to floral tissues or if this KC expression is also present in vegetative tissues of flowering plants prior to and during early floral development. Lastly, CsACO5 has a unique expression pattern in all treatments (uERG; Figure 7), with a significantly larger expression in IMFs compared with MFs or FFs. Here, dosage compensation could play a role with downstream inhibition of ethylene signaling by STS leading to increased transcription of ACO as IMF plants which are genetically female, fail to perceive ethylene levels normally required for female floral development.
Ethylene signaling is conserved in C. sativa
Ethylene signaling has also been highly conserved across the plant kingdom (Chang, 2016). Ethylene binds to a family of receptors known as ETRs, which are found in the membrane of the endoplasmic reticulum of plant cells (Binder, 2020). These receptors are responsible for transducing the ethylene signal to downstream signaling components. The downstream signaling pathway involves a series of conserved components, including CTR1, EIN2, and EIN3, which are responsible for activating the expression of ethylene-responsive genes (Shakeel et al., 2013). In the present study the putative ethylene signaling pathway in C. sativa was identified based on orthology with Arabidopsis genes (Figure 3). Transcriptomes obtained from MFs, FFs and IMFs revealed CsERGs adjacent to, or directly involved in the signaling pathway which have FOC expression (CsETP2, CsFYF2 and CsFYF4) and uERG (CsFYF1). In Arabidopsis EIN2 TARGETING PROTEIN1 and EIN2 TARGETING PROTEIN2 (ETP1/2) act to suppress ethylene responses in the signaling pathway by carrying out ubiquitinating of EIN2, marking EIN2 degradation (Qiao et al., 2009; Binder, 2020). When ethylene is present, ETP1/2 is supressed, allowing ethylene signaling to proceed (Qiao et al., 2009; Yang et al., 2015). In the present study, both CsETP1 and CsETP2 transcripts were detected, however only CsETP2 had an FOC expression associated with sexual plasticity (Figure 3). CsETP2 expression was lowest in FFs compared to MFs and IMFs (Figure 6). There was no significant difference between CsETP2 expression in MFs and IMFs and both had higher expression than FF tissues. These patterns of gene expression fit with the hypothesized role of ethylene as a feminizing agent. If increased endogenous ethylene is responsible for femaleness, then we expect lower levels of CsETP2 in female flower producing plants, as observed.The differential expression analysis also revealed three FOREVER YOUNG FLOWER (FYF) genes which belong to the FOC expression (CsFYF2 and CsFYF4) and uERG (CsFYF1) expression. FYF genes are expressed in young flowers prior to pollination (Chen et al., 2011) and acts as a suppressor of floral abscission. They act by repressing Ethylene Response DNA-binding Factors (EDFs) found downstream ERFs that trigger floral senescence and abscission in maturing flowers (Chen et al., 2015, 2022). In both datasets, expression of CsFYF1 and CsFYF2 was highest in FFs compared with MFs (Supplemental Figure 3) and highest in FFs when compared with IMFs present in dataset 1 (Figure 6 and Figure 7). While FYF expression generally decreases over time in other plants such as orchids or Arabidopsis (Chen et al., 2011, 2021), their expression patterns have not been explored in relation to sex expression in those species. These present expression patterns suggest that FYF may vary with sexual plasticity in dioecious cannabis, but further investigation is needed to determine causality. It is also possible that the discrepancy in CsFYFs levels between FF and other tissues is influenced by the differing lifespans of male and female cannabis flowers. Female cannabis flowers, which have a longer lifespan, may maintain higher levels of CsFYF to suppress senescence caused by ethylene. Exploring endogenous ethylene levels and studying the expression of CsFYF genes throughout flower development could provide more insights into these observed expression patterns.
Among the signaling genes analysed, CsERS1, CsEIL1 and CsEIL3 exhibited KC expression (Figure 5). Both IMF and FF displayed similar, lower levels of CsERS1 expression compared to MFs, indicating a potential association between high CsERS1 expression and male plants in C. sativa. However, it is worth noting that these observations contrast with a previous study by Yamasaki et al. (2000), which reported higher transcript levels of ethylene receptors ETR1, ETR2, and ERS in gynoecious cucumbers compared to monoecious cucumbers, indicating a possible connection between receptor expression and a preference for female flower production. Ethylene receptors have been the subject of extensive study in Arabidopsis, which has five isoforms of ethylene receptors. These are divided into two subfamilies, with ERS1 and ETR1 belongs to the first subfamily and ERS2, ETR2 and EIN4 belonging to the second subfamily (Binder, 2020). Presently no orthologs of AtEIN4 or AtERS2 were identified in cannabis (Figure 3). ETR1 from subfamily 1 has largely been the focus of existing studies on ethylene perception and plant sex determination, with one study finding that ETR1 alone could mediate silver induced inhibitions of ethylene response (McDaniel & Binder, 2012) while another study in Cucurbita pepo found that ETR1 was responsible control for sex determination (García et al., 2020). Liu et al. (2012) also demonstrated that etr1-1(C65Y)Arabidopsis mutants had reduced ethylene responses independently of other receptors, highlighting the significant role of ETR1 in ethylene signaling. However, in both datasets analysed CsETR1 was not differentially expressed in any of the flower types. In contrast, ERS1 which exhibited KC expression in cannabis, has been described to have a modest role as an ethylene receptor in other plants species. Studies suggest that ERS1 may act in coordination with other ethylene receptors to affect signaling (Liu & Wen, 2012) and that its response to silver ions is much smaller than ETR1 (McDaniel & Binder, 2012).
Ethylene insensitive-3 (EIN3)/Ethylene insensitive-3-like (EIL) family is a small family of transcription factors involved in ethylene signaling (Binder, 2020). We found that CsEIL1 and CsEIL3 were differentially expressed between flower types. EILs have been shown to play a role in physiological processes which rely on ethylene, including responses to environmental stresses (Liu et al., 2019), sex determination (Li et al., 2021) and floral development (Zhu et al., 2022). EIN3/EIL1 transcription factors have been implicated with increasing the ratio of female flowers in monoecious pumpkin in response to ethephon application on seedlings (Li et al., 2021) and EIN3/EIL1 expression triggered by ethylene causes developmental defects and pollen sterility in anthers of Arabidopsis. We found higher levels of EIL1 in XX flowers than in those of XY plants, and this raises the question as to whether elevated levels of EIL1 detected in IMFs may affect pollen viability of induced males. In a study which overexpressed mulberry EIL3 in Arabidopsis, the upregulation of EIL3 also coincided with increases in ethylene biosynthesis; however, EIL1 and EIL2 did not follow EIL3 expression under drought and salt stress, suggesting that expression within this family of transcription factors can vary (Liu et al., 2019). These findings are similar to ours, where CsEIL1 showed clear KC expression, with highest levels of expression in FFs and IMFs, whereas CsEIL3 showed slightly more elevated expression in MFs compared to FFs (Figure 5).
Our results highlight the differential expression of CsERS1 and CsEILs between male and female flowers, supporting their involvement in sex determination mechanisms. Future studies should explore the functional significance of ERS1 and EILs in sex determination pathways and investigate the cooperative interactions between ERS1 and other ethylene receptors and downstream signaling to transcription factors in these processes. Moreover, further investigations are warranted to elucidate the specific mechanisms underlying the differential responses to silver ions among receptor subfamilies and their implications for ethylene perception.
Genes linked to sexual plasticity found throughout the genome
C. sativa contains a pair of heteromorphic sex chromosomes. However, treatment of male plants with ethylene and female plants with the ethylene inhibitor STS has been reported to cause plants to produce flowers opposite than that of the sex chromosome karyotype (Green, 2005; Lubell & Brand, 2018; Moon et al., 2020a). The observed sexual plasticity in the species suggests that cannabis sex is not solely determined by the sex chromosomes, but rather by a combination of genetic and environmental factors (GSD+ESD), as has been suggested in the study of mammalian sexual plasticity (Lambert et al., 2019). There are few studies in cannabis which explore the link between sex chromosomes and sex-expression, and to the authors’ knowledge, no studies which explicitly explore molecular mechanisms linking cannabis sexual plasticity and ethylene. In a study comparing the transcriptomes of male and female flowers, Prentout et al. (2020) reported that 35% of sex-linked genes mapped to autosomes while 65% mapped to the sex chromosomes of C. sativa. The distribution of their reported sex-linked genes in the chromosome is in stark contrast to the distribution of CsERGs that we identified with only 26.2% of CsERGs mapping to the sex chromosomes. When considering only CsERGs which are differentially expressed, 20% (3/15) are located on the sex chromosomes, while the remainder are located on the autosomes (Figure 4). The ubiquity of ERGs in the autosomes is to be expected, as they play important roles in general plant growth with ethylene regulating many plant growth responses. Prentout et al. (2020) suggested that their observation of autosomal mapping for sex-linked genes might have been the result of false positives or incorrect placement of genes in the genome assembly of C. sativa. However, their assumption appears to overlook the possibility that many genes involved in plant sex might also be crucial for the production of essential growth regulators or metabolites, which are required in both genetically male and female C. sativa and whose loci are predominantly found on autosomes. In contrast, Adal et al. (2021) proposes an X:autosome dosage mechanism as the basis for sex determination, where male flowers might possess a limited number of unique Y-chromosome genes influencing their sex. We propose that the regulation of cannabis sexual plasticity is mediated by the differential expression of CsERGs, encompassing both FOC and uERG expression, on both autosomes and sex chromosomes of cannabis. The presence of CsERGs on autosomes aligns with ethylene’s involvement in general plant metabolism, while their presence on sex chromosomes suggests some specific roles in sexual development. By considering the comprehensive distribution of CsERGs across the genome, we highlight the importance of both autosomal and sex chromosome-associated genes in the regulation of cannabis sexual plasticity.Limitations and future directions
Based on previous observations suggesting a link between ethylene and sexual plasticity/determinism, the present study constitutes a preliminary analysis to identify putative candidate genes involved in the sexual expression of cannabis. However, in the case of this exploratory study, there are some limitations that we would like to acknowledge. Only two transcriptomic datasets from floral tissues were available that contained multiple flower sexes (Prentout et al., 2020; Adal et al., 2021). In an effort to begin probing the effect of CsERGs during chemically induced sexual plasticity, it was important that some of the transcriptomes include IMFs, which were only available in one of the two datasets used (Adal et al., 2021). Additionally, no existing transcriptomes were available that contained IFFs, which limited our ability to test whether there is an inverse relationship in the mechanisms for sexual plasticity between MFs and IFFs. Future studies on sexual plasticity in cannabis should include IFFs in order to provide a more complete picture of the mechanisms underlying sexual plasticity in the species. Despite these limitations, the differential expression findings between MF and FF in both datasets was used to find a set of differentially expressed CsERGs (Supplemental Figure 1 and Supplemental Figure 3), which are strong candidate CsERGs with a role in sex determination.One limitation of the study is the use of datasets collected at a single timepoint, specifically from flowers at a later stage of development. This approach may result in the potential omission of differentially expressed CsERG candidates that are not active during that specific timepoint, particularly those involved in early floral sex determination or sex differentiation. Furthermore, it is important to acknowledge that some of the identified candidates may exhibit differential expression at the selected timepoint for reasons unrelated to the intended analysis, which aims to establish their role in sexuality. The datasets employed in this study were generated under different experimental conditions, including variations in plant growth conditions, sampling stages of flower development, and sampling locations on the plants. It is crucial to note that the datasets were derived from distinct genotypes of C. sativa: one drug-type (cv. MS-17-338; Adal et al., 2021) and one hemp-type cannabis (cv. Zenitsa; Prentout et al., 2020). As cannabis is known for its high genetic variability (Lapierre et al., 2023), studies based on a single genotype lack the robustness necessary for generalization (Page et al., 2021; Monthony et al., 2021b). Therefore, future studies on C. sativa should aim to include a broader range of cannabis genotypes, particularly those exploring the role of ethylene in plasticity and sex determination. Notably, despite these limitations, the transcriptomes from the two different cannabis populations facilitated the validation of 34 out of the 43 predicted CsERGs listed in Table 1. This validation adds robustness to the gene models proposed in this study for the Yang Cycle, ethylene biosynthesis, and signaling pathways (Figure 2 and Figure 3). It is important to emphasize that the lack of expression of certain predicted CsERGs in both datasets does not necessarily imply that they are non-functional CsERGs. As previously discussed, the inclusion of transcriptomes covering a wider timeline, including early floral induction stages, will contribute to the comprehensive validation of these predicted models.
There are currently a limited number of biotechnological tools (e.g., in vitro culture, CRISPR, VIGS, agrobacterium, etc.) which are optimized in order to functionally validate CsERGs in cannabis (Hesami et al., 2021) and many of these tools have been hampered by the lack of a reliable in vitro regeneration protocol for the species (Monthony et al., 2021b). Although such functional validation is beyond the scope of the current study, it represents an important next step in probing the role of ethylene in cannabis sexual plasticity. Recently progress has been made in establishing methods for gene silencing in cannabis, through the employment of the Cotton leaf crumple virus (CLCrV) in a virus-induced gene silencing protocol reported by Schachtsiek et al. (2019) and through new methods using agrobacterium (Sorokin et al., 2020; Galán-Ávila et al., 2021). However due to their recent nature, these methods have yet to be replicated independently. Given the genotypic variability of cannabis and the role it plays in the replicability of in vitro methods (Monthony et al., 2021b), it remains to be seem how effectively these methods are adapted to multiple genotypes across different research settings.
Conclusion
This study represents the first step in exploring sexual plasticity in the dioecious plant C. sativa and highlights the importance of the Yang cycle, ethylene biosynthesis and signaling pathways in sex determination. Here, we present a framework consisting of three distinct gene expression patterns (KC, FOC and uERG) which can be used to categorize the role of CsERGs in sexual plasticity in dioecious plants. These findings are based on our analysis of transcriptomes of male, female and induced male flowers obtained from two different Cannabis sativa genotypes produced under two different experimental conditions. From the ortholog analysis undertaken in this study and with support from transcriptomic data, we propose that the canonical Yang Cycle and ethylene biosynthesis and signaling pathways are conserved in C. sativa and we identified seven genes belonging to two the FOC and uERG classes which we hypothesize play a role in ethylene-induced sexual plasticity in cannabis. This work lays the groundwork for more detailed study of the role of CsERGs in sexual plasticity in multiple genotypes of cannabis. Given the commercial importance of cannabis, as well as a number of other dioecious plants such as kiwi, papaya and persimmon, understanding the underlying factors of sexual plasticity and its link with ethylene are important to future efforts in breeding and crop improvement.2023.04.28.538750v3.full.pdf
 drive.google.com
drive.google.com
acespicoli
Well-known member
Here, we report the isolation and phenotyping of a monoecious XY cannabis plant that challenges the current understanding of monoecy in Cannabis (XX) and provides a valuable research tool to understand this economically important trait.

Front Plant Sci. 2024; 15: 1412079.
Published online 2024 Jun 6. doi: 10.3389/fpls.2024.1412079
PMCID: PMC11187236
PMID: 38903434
Front Plant Sci. 2024; 15: 1412079.
Published online 2024 Jun 6. doi: 10.3389/fpls.2024.1412079
PMCID: PMC11187236
PMID: 38903434
Why not XY?
Male monoecious sexual phenotypes challenge the female monoecious paradigm in Cannabis sativa L.
acespicoli
Well-known member
Of course everyone is doing their best to select females,
how many people are putting effort into male selections ?
Male studs are highly sought after in the cannabis breeding world. The main reason for that is that it is hard work to find a true breeder male because you cannot directly observe the female traits that a male will pass on to its offspring.
Just by looking at a random man in the street, would you be able to guess his future daughter’s bra size?
Trying to figure out what female attributes a male cannabis plant will pass on to its offspring is almost as difficult.
Fortunately, there are a few things that can increase your likelihood of finding the right male stud.
Let’s find out what they are!

Males that flower the fastest are not necessarily the most desirable. Oftentimes, the males that show sex first, flower too quickly or get too tall have to be culled out. That is because they put more energy into fibre production than flower and cannabinoid production.
Keep in mind that the most accurate way to know whether a male could be a breeder male is by growing out its offspring and observing the females.That being said, it may not always be feasible to grow out the offspring of all your males. It would take up a whole lot a space and be very labor intensive.
This quick tutorial will help breeders narrow down the number of potential male studs so as to save time, money and energy.
Play Video
Here are a few simple techniques breeders use to optimize their probability in order to find the holy grail.
These are the things that may be overlooked by some home breeders. However, it is paramount to avoid these traps in order for your cannabis breeding programs to be successful.

 khalifagenetics.com
khalifagenetics.com
Some valid points in this article
how many people are putting effort into male selections ?
Advanced Cannabis Breeding, Selecting A Male
Male studs are highly sought after in the cannabis breeding world. The main reason for that is that it is hard work to find a true breeder male because you cannot directly observe the female traits that a male will pass on to its offspring.
Just by looking at a random man in the street, would you be able to guess his future daughter’s bra size?
Trying to figure out what female attributes a male cannabis plant will pass on to its offspring is almost as difficult.
Fortunately, there are a few things that can increase your likelihood of finding the right male stud.
Let’s find out what they are!

Pick The Winners
Cannabis breeding is rife with myths and urban legends. In this article, we will go over some useful proven tips to help you pick the winners.Males that flower the fastest are not necessarily the most desirable. Oftentimes, the males that show sex first, flower too quickly or get too tall have to be culled out. That is because they put more energy into fibre production than flower and cannabinoid production.
Keep in mind that the most accurate way to know whether a male could be a breeder male is by growing out its offspring and observing the females.That being said, it may not always be feasible to grow out the offspring of all your males. It would take up a whole lot a space and be very labor intensive.
This quick tutorial will help breeders narrow down the number of potential male studs so as to save time, money and energy.
Desirable traits In The vegetative phase:
Stem Smell
Stem smell is a great indicator. It can give you a pretty accurate idea of what terpene profile a male will pass on to its offspring. On top of that, If you do a stem rub on a young male and get a strong smell, then it would be a great sign, as trichome production and flavor are often related to plants that produce strong aromas early on.Vigor
Vigor is especially important when creating an inbred line (IBL). When doing so, you have to maintain as much vigor as possible while reducing the amount of genetic diversity with each generation to make the strain more and more uniform. In order to optimize your space, you can cull out all the plants lacking vigor early on.Plant Structure And Sturdiness
A male plant that has a very sturdy frame and a short internodal distance is more likely to be a good male for breeding. Likewise, males that have a wide hollow main stem rather than a pith filled stem usually tend to produce higher levels of THC.Desirable Traits During Flowering:
Flower Production
Males that produce dense floral clusters rather than wispy airy ones are usually better breeding material as they tend to produce females that will also develop tight floral clusters.Trichome Production
Some males are covered in trichomes, just like female plants. Not all good male studs produce lots of trichomes but that is always a good sign.Play Video
Increase Your Chances Of Finding The Right Male Plants
Here are a few simple techniques breeders use to optimize their probability in order to find the holy grail.
Reversing Male Plants
It is possible to reverse male plants just as you would female plants. Using an Ethephon spray. Ethephon is a plant growth regulator that acts by causing the release of ethylene. Its toxicity is very low. After spraying Ethephon every day on a target male plant, it will then quickly convert the Ethephon into Ethylene and start producing female flowers. This is very useful in order to gauge what types of qualities (Floral clusters’ structure, smell…) it could potentially pass to the female offspring.Stress-testing The Males
Just as breeders stress test their females in order to only use pure, sexually-stable females in their breeding programs, it is possible to stress test males plants as well. By disturbing the plants’ light cycle, increasing the temperatures and giving too much nitrogen, some males may produce a few female flowers and therefore reveal their hermaphroditic tendencies. Note that it is much easier to stress-test a female than a male plant.Smoking The Males
Some breeders like to smoke their male plants in order to know which plants have the best potential. Because male plants generally have low-levels of cannabinoids, it is not easy to do an accurate smoke test. That being said, it is one more trick you can use in order to make the best choice.Lab-testing The Males
Lab testing several male plants can be costly but it is by far the most accurate method. It will give you the exact cannabinoid level and terpene profiles of your males. By using some of the tips and tricks aforementioned, you can greatly narrow down the number of males you will have to lab-test.Avoid The Traps:
These are the things that may be overlooked by some home breeders. However, it is paramount to avoid these traps in order for your cannabis breeding programs to be successful.
Separating each male (Pollen contamination)
Before using a male for pollinating a female, isolate that male for a few days in an individual grow tent/grow box. Since pollen flies everywhere, leaving a male plant amidst other males up to the pollination day will result in pollen contamination.Let The Males Finish
Some home breeders pick their favorite males before the end of their flowering cycle. Let them go to the end of their flowering cycle just as you would with females. These males may express some specific traits at the very end of their life cycle. Which would help you make the decision as to which males you are going to want to keep.A Head Start In Cannabis Breeding
Knowing which males to pick takes time. New breeders will have to develop the habit and learn from their experiences. However, this practical guide will give a good head start in your future cannabis breeding endeavors!
Advanced Cannabis Breeding, Selecting the Best Male - Khalifa Genetics
Cannabis breeding is rife with myths and urban legends. In this article, we will go over some useful proven tips to help you pick the best breeder male.
 khalifagenetics.com
khalifagenetics.com
Some valid points in this article
acespicoli
Well-known member
- Home
- Blog
- Cultivation
- How to Select the Best Male Cannabis Plants for Breeding
How to Select the Best Male Cannabis Plants for Breeding

By Sera Jane Ghaly
Updated on 08/28/2023
Share
You should pride yourself on being very choosy when it comes to selecting a male – a male cannabis plant that is. Actually, the future of your cannabis breeding project rests on your ability to discern good genetics from bad genetics. In this article, you can learn which things to look out for to successfully choose a male plant!
Until just a few decades ago, choosing a male was left up to natural selection.
But these days, we get to play an active role in which genetics go on into the future.
And no, I’m not talking about a lady’s preferences when choosing a mate. This article is about carefully selecting a male cannabis plant in the process of breeding and creating seeds. Sorry to disappoint those looking for a husband.
The reason selecting a male cannabis plant is an art is because breeders are essentially mimicking nature. Taking the place of natural selection is a big responsibility! If the entire future of your cannabis garden relied on it, you would hope to choose a good male, right? So… how exactly does one do that? I assure you, it’s kind of like choosing a man.
By carefully choosing which male genetics you are going to use to make seeds, you can be well on your way to creating badass cannabis strains in your very own garden. So, let’s get to business and find out what to look out for when you’re choosing the father of your cannabis offspring!
1. Ideally, you’ve already started breeding
Before you start making your own seeds, you have ideally created your own first-generation hybrid strain. If you’re not up to that yet, you do that by crossing two great strains (ideally pure indica and pure sativa).Hopefully, what you’ve created is consistent in phenotypic expression. However, in this stage it’s common to see a lot of variation. It’s in the next generations that as a breeder, you get to carefully select the phenotypes that you want to express.
Keep in mind that while phenotype is important during the growing process, you don’t actually know what your buds are like until after you’ve harvested – because that’s when you get to smoke them. For this reason, it’s good not to get ahead of yourself. Big buds and a delicious smell are nice. But if in the end, it’s a boring smoke, then has your selection really been worthwhile?
While the female plants are the ones you will finally sample, the male cannabis plants play a huge role in what you eventually get to experience. So, it can be a little bit involved and complicated to choose a male.
Ideally, your first-generation hybrid gave you a big batch of seeds to start off with, and that gives you a lot of choices during the second generation. And trust me, you will get better and better at selecting by third, fourth and fifth generation of your own hybrid strain.

2. Save some for later!
As mentioned, you only really know if you’ve selected the best parents after the future generation of seeds has been planted, harvested, dried and cured, and smoked. That’s a whole season since you decided on that male and that female! So paradoxically, we’re working a little bit in reverse to see if you’ve been a good substitute for natural selection.Save some samples of each plant that you’ve decided to keep and “test”. You can do this by keeping cuttings and making rooted clones. Then, you can re-green the mother or the father (or both) and keep it in a vegetative state.
This allows you the possibility of going back to that parent plant after harvesting its progeny. Alternatively, you can collect some pollen, vacuum seal it, and put it in the freezer. This is for serious genetic seekers!
So now that you’ve got some tips and tricks up your sleeve, here are some things to look out for when it comes to actually choosing the male cannabis plant.
Related post

Cultivation
The Flowering Stage Of Cannabis Plants: Tips for the Best Buds
Kenny Hall29 Jul 2024
3. Let go of early-bloomers
Unless you’re trying to breed a plant that flowers early and fast, get rid of male plants that show this trait. Just on a side note, knowing what you actually want from your strains before selecting the male is extremely helpful.Males that flower before you’ve flipped them to flower means they are naughty and don’t listen! Just kidding. It means that they are possibly prone to unwanted traits when it comes to flowering.
So, to ensure quality and avoid hermaphroditism, remove any early bloomers (but don’t throw them away!). They aren’t really selection-worthy.
Related post

Cultivation
Topping Cannabis: A Hack for Bushier Plants and Bigger Yields
Julien10 May 2023
4. Don’t be fooled by height
Tall, dark and handsome. They are the dreamy desires of every woman, right? Well, not in the case of Mary-Jane.Plants that dedicate a lot of energy to producing the fibres that make them tall are probably better left for producing fibres, not buds!
It is better to eliminate male plants that grow too tall and too quickly. Unless they have other characteristics that you find very appealing, leave them out of the equation.
Related post

Cultivation
How to Water Cannabis Plants: Techniques, Tips, and Tricks for Optimal Hydration
Julien29 Apr 2024
5. It’s all in the stems
This piece of advice is borrowed from Michael Stark’s book, Marijuana Chemistry, and it has been backed up by many breeders. What you’re looking for in the stem of a male plant is hollowness.Yep, depth of character isn’t important in growing weed. A thick, dense and full main stem should be eliminated. Michael Starks (and many breeders) observe that a hollow stem is somehow linked to THC production.
For a more in-depth analysis of the chemistry and genetics there, you should probably read his book!

6. Males make flowers, too
Yes, male plants make flowers, too! Just not the kind that we finally smoke. However, the pollen that forms in these flowers is what you finally use to pollenate your females.Observe the way that flowers have developed on your male plants. At the end of the day, you don’t want to pass on the genetics of a very whispy or unsubstantial bud. You want it dense and thick, just like buds should be! The writing is on the wall when it comes to the female plant, but it’s a bit trickier to tell with the males because we generally aren’t used to looking at their flowers.
You also want to inspect the buds of the male plant for mildew or mould. If the particular male is prone to mould or mildew, let it go. You want to produce robust seeds that are resistant towards these types of deadly problems.
You will notice that some of the male plants in your garden are somehow more susceptible than others. Let it be their natural end.
Related post

Cultivation
Lollipopping Cannabis Plants For Maximum Yield: Less is More
Kenny Hall06 Aug 2024
7. Do you scent something special?
Let your observation of male plants be a very scentual experience. The strength of the odour during the vegetative state should tell you a lot about its THC production. Keep plants that have a strong, delicious smell that you like. Let go of the ones that are weaker in odour.The best way to test for the smell of a male cannabis plant is by rubbing the leaves and the stem. How much of a scent does it release? Can you still smell it on your hands even after you walk away from it?
8. Trichomes
Male plants don’t put on the light show of a female plant when it comes to trichomes, but they do exist. Inspect them. Just as you would with female plants, choose the males that have many trichomes! The potency of the male plant should contribute to the potency of its offspring, right?Related post

Cultivation
The Seedling Stage Of Cannabis Plants: A Week-by-Week Guide
Kenny Hall19 Jul 2024
9. The more seeds you grow, the better
To get the best out of your breeding venture, grow as many seeds as possible. The more you grow, the more phenotypes you will be able to observe, and consequently, the bigger your selection of choices.It is unusual to find that special one, you know, so it’s better to keep your options open. The more seeds you plant, the more likely you are to find that extra special male plant.
Now, if you happen to have a male plant that shows all the desired traits, then you’re a very lucky breeder, indeed.
But that doesn’t usually happen in the first or second generations. Rather, your careful selection begins to show itself over the coming generations, when your plants more consistently show the same phenotypes and keep producing great seeds.
Before long, you’ll have a master strain that everybody will be after!
I have been following Khalfia for quite awhile now, they are very responsive and Friendly to work with. The lemon selection they worked up from the Balkhi Line was interesting and im hopeful they continue those endeavors for some of their other landrace genetics. Maybe their male selections have something todo with the quality breeding im not seeing elsewhere



