Maximizing Cannabis Yields with Dr Bruce Bugbee
Apogee Instruments Inc.
The first thing I did was to convert specific gravity (SG in 1.xxx format) to degrees Plato (°P) with the equation
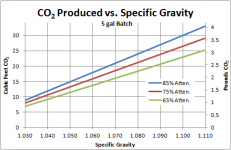
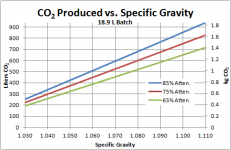
To find the mass of sugar in each wort, I then found the weight of a 5 gal/ 18.9 L batch by multiplying this volume by its density (if working in metric, this amounts to simply multiplying by the specific gravity since water is 1 kg/L). Then, its a simple matter of multiplying by the sugar weight percent. The example 1.060 wort contains (44.23 lb wort)*(0.14741)= 6.52 lb or 2.96 kg sugar. Note that I actually did all of my calculations in metric, then converted to English because the units work out so nicely.
Next, we must find how much sugar is actually consumed. For this, remember that the “attenuation” used most often is “apparent attenuation” as measured by a hydrometer. Hydrometers actually measure the density of the solution, so when alcohol is created (its density is lower than water), the hydrometer is tricked into thinking more of the sugar has been consumed than really was. Actual attenuation can by found by multiplying the apparent attenuation by 0.814, as given in Greg Noonan’s New Brewing Lager Beer. So the actual amount of sugar consumed in a 1.060 75% apparent attenuation beer is (6.52 lb)(0.75*0.814)= 3.98 lb or 1.81 kg.
Now we must bring in some very basic chemistry. Lets assume this sugar is all (or could become) glucose. A single mole (in units of mol) of glucose weighs 180.156 g or 6.35 oz. A mole is simply a way to keep track of how many molecules of something there is so you can predict chemical reactions. Basically, there are a different number of molecules of acid in vinegar in a gram than the number of baking soda molecules in a gram, but reactions happen to molecules. So to have a complete reaction, you need to add the same number of molecules of acid in vinegar and baking soda, not the same number of grams of each. The upshot is we have (1.81 kg)/(0.180156 kg/mol)=10.02 mol glucose consumed in this example wort.
For every 1 mol glucose consumed, 2 mol of ethanol and 2 mol of CO2 are produced. Thus we have 10.02*2=20.04 mol of total CO2 produced from fermenting this 1.060 wort to 75% apparent attenuation.
Now we can put this in terms of volume or mass of gas. For any “ideal” gas, its volume can be predicted by the ideal gas law at standard temperature and pressure,
To find the mass of the gas, we simply use the mass of a mole of CO2, 0.04401 kg/mol, to convert back. We have (20.04 mol)*(0.04401 kg/mol)=0.88 kg or 1.94 lb of CO2 produced.
– Dennis,
Life, Fermented

How Much CO2 is Produced from Brewing?
Off-week bonus post! Ever wonder how much CO2 is actually produced when you ferment your favorite bubbly beverage? I’ve often found myself staring at my air-lock or blow-off bucket thinking…
Attachments
Last edited: