TH was the inspiration for haystack title
@Tom Hill 5% not hay

Tom Hill Haze
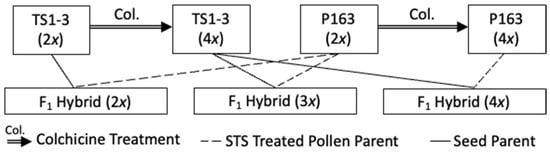
Hi All, I've been asked a few times, so I will just say for the record - the Tom Hill seed was produced under Tom's direction in Spain - it's the same seed you have seen elsewhere on the market, it's just been in the scientific seed fridge since it arrived to me shortly after it was produced...
www.icmag.com
Last edited:




 10% maybe less...
10% maybe less...