Detailed Explanation of How the Atmosphere Works
Detailed Explanation of How the Atmosphere Works
To go along with the previous post...
Sorry this is a bit long but, necessarily so...
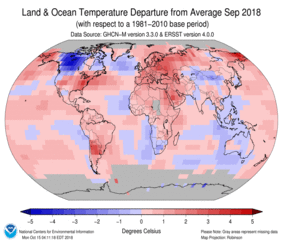
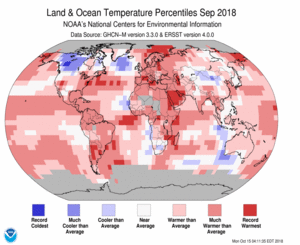
Each part of the Earth’s surface emits heat in the form of infrared (IR) radiation. The peak of this emission is right at the frequency where CO2 absorbs strongly. While the proportion of CO2 in the atmosphere is small, 410 parts per million or 0.041%, this is still a large number of molecules, large enough that near the surface, at wavelengths where CO2 absorbs, the average distance light will travel before being captured is a few meters (a couple of yards).
Greenhouse gases, as well as absorbing IR radiation, emit it. Almost none of the greenhouse gas molecules that absorb IR light emit it immediately. Instead the internal excited energy of the molecule is transformed into thermal motion of
molecules nearby through collisions. This takes about a microsecond, a millionth of a second and is roughly a million times more likely than the molecule directly emitting IR light.
In the same way unexcited greenhouse gas molecules can be excited by collisions into a state where they can emit. It turns out that the rate at which excited molecules can form increases with temperature.
The distance that the emitted radiation can travel is short near the surface, but increases as one climbs through the atmosphere because density, pressure and temperature decrease as we climb. Each of these lengthens the distance radiation emitted from a molecule travels before being absorbed, until about at 10 km altitude where the temperature is -50 C (or ~-60 F or~220 K) and the density has decreased by a factor of ~3, it becomes possible for radiation from CO2 molecules to reach space, carrying thermal energy away from the Earth. Below that level, energy emitted by a CO2 molecule is soon absorbed by another relatively nearby one. Thus this energy simply cannot be radiated to space to balance the incoming solar energy.
Taken together this means that the doorway to space is very narrow at wavelengths where CO2 can absorb. Since the same amount of energy has to be radiated to space as is coming from the sun, something has to increase, and that is the temperature of the surface. As the surface warms, the rate at which it can radiate energy increases, pushing more thermal IR radiation out into space in spectral regions where ghg molecules don’t absorb.
If we increase the proportion of CO2 in the atmosphere, the altitude at which energy can be radiated to space rises also, but since this higher level is colder and the pressure and density are lower, the doorway becomes narrower, and the surface has to warm more in order to shove the same amount of energy out and restore the balance with the incoming energy carried by the sunlight.
Figures can be seen at*https://rabett.blogspot.com/2010/03/simplest-explanation.html
Detailed Explanation of How the Atmosphere Works
To go along with the previous post...
Sorry this is a bit long but, necessarily so...
Each part of the Earth’s surface emits heat in the form of infrared (IR) radiation. The peak of this emission is right at the frequency where CO2 absorbs strongly. While the proportion of CO2 in the atmosphere is small, 410 parts per million or 0.041%, this is still a large number of molecules, large enough that near the surface, at wavelengths where CO2 absorbs, the average distance light will travel before being captured is a few meters (a couple of yards).
Greenhouse gases, as well as absorbing IR radiation, emit it. Almost none of the greenhouse gas molecules that absorb IR light emit it immediately. Instead the internal excited energy of the molecule is transformed into thermal motion of
molecules nearby through collisions. This takes about a microsecond, a millionth of a second and is roughly a million times more likely than the molecule directly emitting IR light.
In the same way unexcited greenhouse gas molecules can be excited by collisions into a state where they can emit. It turns out that the rate at which excited molecules can form increases with temperature.
The distance that the emitted radiation can travel is short near the surface, but increases as one climbs through the atmosphere because density, pressure and temperature decrease as we climb. Each of these lengthens the distance radiation emitted from a molecule travels before being absorbed, until about at 10 km altitude where the temperature is -50 C (or ~-60 F or~220 K) and the density has decreased by a factor of ~3, it becomes possible for radiation from CO2 molecules to reach space, carrying thermal energy away from the Earth. Below that level, energy emitted by a CO2 molecule is soon absorbed by another relatively nearby one. Thus this energy simply cannot be radiated to space to balance the incoming solar energy.
Taken together this means that the doorway to space is very narrow at wavelengths where CO2 can absorb. Since the same amount of energy has to be radiated to space as is coming from the sun, something has to increase, and that is the temperature of the surface. As the surface warms, the rate at which it can radiate energy increases, pushing more thermal IR radiation out into space in spectral regions where ghg molecules don’t absorb.
If we increase the proportion of CO2 in the atmosphere, the altitude at which energy can be radiated to space rises also, but since this higher level is colder and the pressure and density are lower, the doorway becomes narrower, and the surface has to warm more in order to shove the same amount of energy out and restore the balance with the incoming energy carried by the sunlight.
Figures can be seen at*https://rabett.blogspot.com/2010/03/simplest-explanation.html
Last edited: