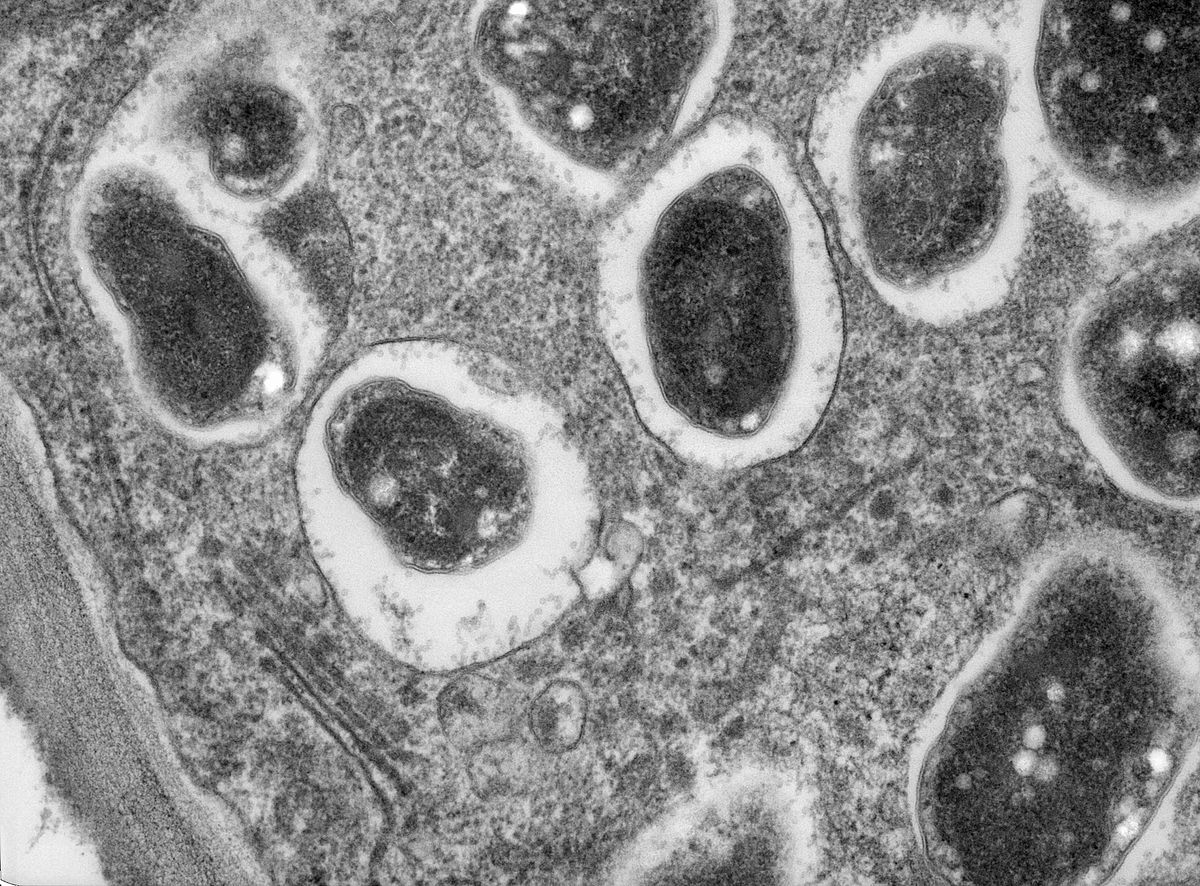
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient.
flux=−�(�2−�1)

in which
The exchange rate of a gas across a fluid membrane can be determined by using this law together with Graham's law.
Under the condition of a diluted solution when diffusion takes control, the membrane permeability mentioned in the above section can be theoretically calculated for the solute using the equation mentioned in the last section (use with particular care because the equation is derived for dense solutes, while biological molecules are not denser than water):[12]

where
Biological perspective[edit]
The first law gives rise to the following formula:[19]flux=−�(�2−�1)

in which
- P is the permeability, an experimentally determined membrane "conductance" for a given gas at a given temperature.
- c2 − c1 is the difference in concentration of the gas across the membrane for the direction of flow (from c1 to c2).
The exchange rate of a gas across a fluid membrane can be determined by using this law together with Graham's law.
Under the condition of a diluted solution when diffusion takes control, the membrane permeability mentioned in the above section can be theoretically calculated for the solute using the equation mentioned in the last section (use with particular care because the equation is derived for dense solutes, while biological molecules are not denser than water):[12]

where
-
is the total area of the pores on the membrane (unit m2).
-
transmembrane efficiency (unitless), which can be calculated from the stochastic theory of chromatography.
- D is the diffusion constant of the solute unit m2⋅s−1.
- t is time unit s.
- c2, c1 concentration should use unit mol m−3, so flux unit becomes mol s−1.