Deficiency Pathology - Diagnose Sick Plants, Follow up with Cure
Using Integrated Pest Management in Greenhouses and Herbaceous Nurseries
James Quinn
Horticulture Extension Specialist
David Trinklein
Division of Plant Sciences
Raymond A. Cloyd
Ornamental Entomology/Integrated Pest Management
Kansas State University
For more than 30 years the U.S. Department of Agriculture has promoted integrated pest management (IPM) as a way of dealing with arthropod (insect and mite) pests in greenhouses and herbaceous nurseries. IPM strategies include the use of cultural, biological and physical (or mechanical) methods as well as pesticides to manage pests. IPM relies on routine inspection, scouting and monitoring of arthropod populations followed by the use of insecticides or miticides only when pest populations are capable of causing plant damage. If the use of these pesticides is warranted, then it is important to choose those products that are less harmful to the environment and to beneficial insects and mites (Figure 1).
This publication is designed to assist greenhouse and nursery managers in selecting the appropriate pesticides to control or regulate the multitude of arthropod pests encountered in greenhouses and nurseries. The primary arthropod pests encountered in greenhouses and herbaceous nurseries in both Missouri and Kansas are aphids, thrips, fungus gnats, shore flies, spider mites, mealybugs, plant bugs, whiteflies, leafhoppers, leafminers, leaf-feeding beetles and caterpillars.

Ladybird beetles, known for their appetite for aphids, occur naturally in Missouri but also can be introduced as biocontrol agents in greenhouses and nurseries.
(opens in new window)Ways to reduce use of pesticides
Although pest control materials are generally effective in killing arthropod pests, overreliance on this control method increases the likelihood that resistance will develop in arthropod pest populations. Therefore, it is important to use cultural, physical and biological control strategies as well as using pest control materials. The following practices will reduce the use of pest control materials in greenhouses and nurseries:- Start the growing season with clean greenhouses and nurseries; remove weeds and all other plant material and eliminate debris, such as growing medium.
- Maintain adequate sanitation and use proper cultural practices (watering and fertility) throughout the growing season.
- Scout plants weekly, especially indicator plants, those that typically affected by arthropod pest problems.
- Use colored sticky cards (yellow or blue) and visually inspect plants. Record insect and mite pest observations, such as abundance (number of pests per plant or row) and life stages (eggs, nymphs or larvae, pupae and adults).
- Inspect transplants or propagation material carefully. Isolate newly introduced plants and inspect for any arthropod pest problems. If arthropod pests are present, then treat with an appropriate pest control material.
- Treat only those plants directly affected by arthropod pests or localized infestations.
- If possible, install insect screening over greenhouse openings such as ridge vents, sidewalls and intake vents. Be sure to compensate for the resulting reduction in airflow by increasing the screening surface area.
(opens in new window)Alternative or "reduced-risk" pesticides
Use of pesticides has changed dramatically since 1985. Before that time, pesticides in three chemical classes — organophosphates, carbamates and chlorinated hydrocarbons — were relied upon to manage plant-feeding insects and mites. Use of materials in these older chemical classes was reduced somewhat with the availability of products in a fourth chemical class, pyrethroids. Since 1985, the Environmental Protection Agency (EPA) has reevaluated the registration of older pesticides and has encouraged the development of alternative pesticides that reduce risk to human health, toxicity to nontarget organisms, and the potential for groundwater contamination. These materials are preferred for use in greenhouses and herbaceous nurseries because they are- Less persistent (shorter residual activity) in the environment
- Less directly harmful to natural enemies, including parasitoids and predators
- Effective in controlling arthropod pests at reduced application rates when compared with other pesticides
Tables 1 and 3 list pesticides registered for use in greenhouses and herbaceous nurseries. Table 2 lists those specifically designated for use in organic cropping systems. More information on reduced-risk materials (PDF) is available online at http://epa.gov/opprd001/workplan/completionsportrait.pdf(opens in new window).
New pesticides are generally registered more rapidly for use on ornamental plants than on food crops because they are not edible and do not require extensive food safety testing. However, registration for greenhouse-grown vegetables is usually delayed or may not occur. This may be confusing especially with regard to vegetable bedding plants. Several of the pesticides listed in Table 1 may be used on vegetable bedding plants. However, it is critical to read the label to obtain this information. Higher infestation levels of arthropod pests are more tolerable in vegetable production systems than in ornamental crops because plants such as tomatoes and cucumbers are primarily grown for fruit production, and may even be saleable if the plants exhibit damage from insect or mite pest feeding. Overall, it is important to read the product label before applying any pesticide to make sure that the insect or mite pests as well as the treatment site are designated.
(opens in new window)Guidelines for implementing a biological control program
- Scout the crop regularly to detect early infestations of arthropod pests before they reach damaging levels.
- Order natural enemies early (at least three weeks before they are needed) and release them immediately or as soon as possible after arrival. Follow the supplier’s instruction for release.
- Install insect screening over greenhouse openings such as ridge vents, sidewalls and air intake vents to reduce the migration of winged aphids, adult whiteflies, thrips, and leafminers into greenhouses. Be sure to compensate for any reduction in airflow by increasing the screening surface area.
- Avoid overfertilizing plants, particularly with nitrogen-based fertilizers, because this results in the production of soft, succulent growth that is more susceptible to aphids and the twospotted spider mite (Tetranychus urticae).
- Remove yellow sticky cards before applying parasitoids, because sticky cards can attract and capture parasitoids. Yellow sticky cards can be replaced one week after making releases.
- Reduce the use of pesticide when bumblebees are used as pollinators, and avoid applying pest control materials with extended residual activity, such as products in the organophosphate, carbamate and pyrethroid chemical classes. Drenching applications of systemic insecticides to the growing medium will be less harmful than foliar applications.
(opens in new window)Biological control
Biological control agents, or natural enemies such as parasitoids and predators, can be purchased from commercial suppliers or distributors and released into greenhouses. This practice is referred to as augmentative biological control, for which there are two control strategies: inoculation and inundation. Inoculation consists of releasing small numbers of natural enemies early in the growing season or cropping cycle so that a population of natural enemies will establish and reproduce in the greenhouse, providing long-term control. Inundation is the introduction of much larger numbers of natural enemies into a greenhouse to provide control in the short term. Additional releases may be required during the growing season or cropping cycle to keep arthropod pest populations at low levels.Consult biological control suppliers and distributors for additional information on the use of natural enemies in greenhouses and herbaceous nurseries. Biological control programs tend to be more effective when crops are grown for extended periods (e.g., cut flowers and vegetables) and when environmental conditions (e.g., temperature and relative humidity) are constant. Preventive releases of natural enemies are more efficient and easier in a monoculture (e.g., single crop) cropping system when there is only one arthropod pest than in a polyculture (e.g., multiple crops) cropping systems where there may be more than three different arthropod pests. For example, in the production of spring bedding plants, various insect pests may be present simultaneously, including aphids, thrips, whiteflies and fungus gnats.
The greenhouse environment does not contain the abundance and diversity of natural enemies found in outdoor settings or nurseries. This is mainly because of the extensive use of pesticides and because natural enemies typically do not migrate into greenhouses. The survival of natural enemies in a greenhouse is influenced by the abundance and types of prey that are present. However, certain parasitoids and predators sometimes occur naturally in greenhouses. For example, parasitoids in the genus Aphidius, which prey upon many different types of aphids, can inadvertently enter greenhouses through doors, vents or sidewalls. Adult females lay eggs into aphids, and these eggs hatch into larvae that consume the internal organs of the aphids, leaving only their hardened, brown exteriors, or “aphid mummies” (Figure 2). Eventually, a new adult parasitoid creates an exit hole and emerges from the dead aphid. Minute pirate bugs, Orius spp., are predatory anthocorid bugs that feed on thrips. These black and white bugs may also enter greenhouses through openings, particularly when weeds and field crops start desiccating.
Natural enemies that may be present in outdoor nurseries include ladybird beetles, green lacewings, ground beetles, soldier beetles, assassin/ambush bugs, damsel bugs, hover (syrphid) flies, tachinid flies, predatory mites and spiders.


The Aphidius wasp, left, stings the aphid and lays an egg in the aphid's body, which mummifies, right, as the egg develops.
Marion Herbert, Alberta Research Station, Vegreville, photo
Table 1Pesticides (insecticides and miticides) registered for use on ornamental plants or greenhouse-grown vegetables. (Always read the label to determine if a pesticide can be used in a particular facility and on a specific crop.)
| Common name or active ingredient (Trade name) | Class | Mode of action | Reentry interval | Labeled pests | Additional products |
|---|
| abamectin (Avid) | Macrocyclic lactone | Gamma-aminobutyric acid (GABA) chloride channel activator [6] | 12 hours | spider mites, thrips, leafminers | |
| acephate (Orthene/Precise) | Organophosphate | Acetylcholine esterase inhibitor [1B] | 24/12 hours | aphids, whiteflies, scales, mealybugs, thrips | |
| acequinocyl (Shuttle) | Napththoquinone | Mitochondria electron transport inhibitor [20B] | 12 hours | spider mites | |
| acetamiprid (TriStar) | Neonicotinoid | Nicotinic acetylcholine receptor disruptor [4A] | 12 hours | aphids, whiteflies, mealybugs, scales | |
| azadirachtin (Azatin/Ornazin) | Botanical (insect growth regulator) | Ecdysone antagonist [18B] | 4/12 hours | aphids, fungus gnat larvae, thrips, whiteflies, caterpillars | Aza-Direct and Neemix |
| Bacillus thuringiensis spp. israelensis (Gnatrol) | Microbial | Midgut membrane disruptor [11A1] | 4 hours | fungus gnat larvae | |
| Bacillus thuringiensis spp. kurstaki (Dipel) | Microbial | Midgut membrane disruptor [11B2] | 4 hours | caterpillars | |
| Beauveria bassiana (BotaniGard) | Microbial (entomopathogenic fungi) | Direct infection of host by hyphae | 4 hours | aphids, mealybugs, whiteflies | Naturalis and Mycotrol |
| bifenazate (Floramite) | Carbazate | Gamma-aminobutyric acid (GABA) gated antagonist [25] | 4 hours | spider mites | |
| bifenthrin (Talstar/Attain) | Pyrethroid | Sodium channel blocker [3] | 12 hours | aphids, caterpillars, fungus gnat adults, mealybugs, scales, plant bugs, thrips, leafhoppers, whiteflies | |
| buprofezin (Talus) | Benzoylurea (insect growth regulator) | Chitin synthesis inhibitor [16] | 12 hours | whiteflies, mealybugs, scales and leafhoppers | |
| chlorfenapyr (Pylon) | Pyrrole | Oxidative phosphorylation uncoupler [13] | 12 hours | spider mites, broad mite, cyclamen mite, fungus gnat larvae, thrips | |
| chlorpyrifos (DuraGuard) | Organophosphate | Acetylcholine esterase inhibitor [1B] | 24 hours | aphids, caterpillars, fungus gnat larvae, leafhoppers, mealybugs, shore fly larvae, thrips | |
| clarified hydrophobic extract of neem oil (Triact) | Botanical | Suffocation or membrane disruptor | 12 hours | aphids, whiteflies, spider mites, scales | |
| clofentezine (Ovation) | Tetrazine | Growth and embryogenesis inhibitor [10A] | 12 hours | spider mites | |
| cyfluthrin (Decathlon/Tempo) | Pyrethroid | Sodium channel blocker [3] | 12 hours | aphids, caterpillars, fungus gnat adults, mealybugs, scales, thrips, whiteflies | |
| cyromazine (Citation) | Triazine (insect growth regulator) | Chitin synthesis inhibitor [17] | 12 hours | fungus gnat larvae, shore fly larvae, leafminers | |
| diflubenzuron (Adept) | Benzoylurea (insect growth regulator) | Chitin synthesis inhibitor [15] | 12 hours | fungus gnat and shore fly larvae | |
| dinotefuran (Safari) | Neonicotinoid | Nicotinic acetylcholine receptor disruptor [4A] | 12 hours | aphids, whiteflies, scales, leafminers, thrips, leafhoppers, mealybugs | |
| etoxazole (TetraSan) | Diphenyloxizoline derivative (mite growth regulator) | Chitin synthesis inhibitor [10B] | 12 hours | spider mites | |
| fenbutatin-oxide (ProMite) | Organotin | Oxidative phosphorylation inhibitor [12B] | 48 hours | spider mites | |
| fenoxycarb (Preclude) | Carbamate (insect growth regulator) | Juvenile hormone mimic [7B] | 12 hours | aphids, caterpillars, leafminers, mealybugs, scales, thrips, whiteflies | |
| fenpropathrin (Tame) | Pyrethroid | Sodium channel blocker [3] | 24 hours | caterpillars, fungus gnat adults, mealybugs, whiteflies | |
| fenpyroximate (Akari) | Phenoxypyrazole | Mitochondria electron transport inhibitor [21] | 12 hours | spider mites | |
| flonicamid (Aria) | Trifluoromethylnicotinamide | Selective feeding blocker [9C] | 12 hours | aphids, thrips, whiteflies | |
| fluvalinate (Mavrik) | Pyrethroid | Sodium channel blocker [3] | 12 hours | aphids, fungus gnat adults, thrips, leafhoppers, caterpillars, plant bugs, whiteflies | |
| hexythiazox (Hexygon) | Carboxamide | Growth and embryogenesis inhibitor [10A] | 12 hours | spider mites | |
| imidacloprid (Marathon/Merit) | Neonicotinoid | Nicotinic acetylcholine receptor disruptor [4A] | 12 hours | aphids, whiteflies, scales, mealybugs | Admire, Benefit, Mantra |
| kinoprene (Enstar II) | Insect growth regulator | Juvenile hormone mimic [7A] | 4 hours | aphids, fungus gnat larvae, mealybugs, scales, thrips, whiteflies | |
| methiocarb (Mesurol) | Carbamate | Acetylcholine esterase inhibitor [1A] | 24 hours | aphids, thrips, snails/slugs | |
| milbemectin (Ultiflora) | Macrocyclic lactone | Gamma-aminobutyric acid (GABA) chloride channel activator [6] | 12 hours | spider mites | |
| novaluron (Pedestal) | Benzoylurea (insect growth regulator) | Chitin synthesis inhibitor [15] | 12 hours | thrips, whiteflies, caterpillars, leafminers | |
| paraffinic oil (Ultra-Fine Oil) | Refined petroleum distillate | Suffocation or membrane disruptor | 4 hours | aphids, mealybugs, scales, spider mites, whiteflies | |
| petroleum oil (PureSpray Green) | Refined petroleum distillate | Suffocation or membrane disruptor | 4 hours | aphids, mealybugs, scales, spider mites, whiteflies | |
| potassium salts of fatty acids (insecticidal soap/M-Pede) | Insecticidal soap | Desiccation or membrane disruptor | 12 hours | aphids, caterpillars, fungus gnat adults, leafhoppers, mealybugs, scales, spider mites, whiteflies | |
| pymetrozine (Endeavor) | Pyridine (Azomethine) | Selective feeding blocker [9B] | 12 hours | aphids and whiteflies | |
| pyridaben (Sanmite) | Pyridazinone | Mitochondria electron transport inhibitor [21] | 12 hours | spider mites and whiteflies | |
| pyriproxyfen (Distance) | Pyridine (insect growth regulator) | Juvenile hormone mimic [7C] | 12 hours | fungus gnat and shore fly larvae, scales, whiteflies | |
| pyrethrin (Pyganic) | Botanical | Sodium channel blocker [3] | 12 hours | aphids, caterpillars, beetles, mealybugs, thrips, whiteflies | Pyreth-It and Pyrethrum |
| pyrethrin and silicon dioxide (Diatect V) | Botanical | Central nervous system disruptor and desiccant [3] | 12 hours | aphids, caterpillars, whiteflies | |
| spinosad (Conserve/Entrust) | Spinosyn | Nicotinic acetylcholine receptor agonist [5] | 4 hours | caterpillars, thrips, leafminers | |
| spiromesifen (Judo) | Tetronic acid | Lipid biosynthesis inhibitor [23] | 12 hours | spider mites, broad mite, whiteflies | |
| Steinernema feltiae (Nemasys) | Biological control (entomopathogenic nematode) | Penetrant through insect cuticle and degrades internal contents | 0 hours | fungus gnat larvae | NemaShield, Scanmask, Entonem |
| thiamethoxam (Flagship) | Neonicotinoid | Nicotinic acetylcholine receptor disruptor [4A] | 12 hours | aphids, whiteflies, mealybugs, scales |
Numbers and letters in brackets [xx] indicate the IRAC (Insecticide Resistance Action Committee) mode of action designation found on the label.
Table 2
Pesticides (insecticides and miticides) registered for use in organic production systems (ornamental plants, vegetables and herbs).
| Common name or active ingredient (Trade name) | Class | Mode of action | Reentry interval | Labeled pests |
|---|---|---|---|---|
| azadirachtin (Azatrol/Neemix) | Botanical (insect growth regulator) | Ecdysone antagonist [18B] | 4/12 hours | aphids, fungus gnat larvae, thrips, whiteflies, caterpillars |
| Bacillus thuringiensis spp. israelensis (Gnatrol) | Microbial | Midgut membrane disruptor [11A1] | 4 hours | fungus gnat larvae |
| Bacillus thuringiensis spp. kurstaki (Dipel) | Microbial | Midgut membrane disruptor [11B2] | 4 hours | caterpillars |
| clarified hydrophobic extract of neem oil (Triact) | Botanical | Suffocation or membrane disruptor | 12 hours | aphids, whiteflies, spider mites, scales |
| horticultural oils: petroleum oils (PureSpray Green), plant-based oils (GC-Mite/Golden Pest Spray Oil), fish-based oils (Organocide) | Refined petroleum distillate and botanical | Suffocation or membrane disruptor (some products have multiple modes of action; refer to label). | 4 hours | aphids, mealybugs, scales, spider mites, whiteflies |
| kaolin clay (Surround) | Protectant | Multiple modes of action (refer to label) | 4 hours | caterpillars, beetles, tarnished plant bug, stink bug, thrips |
| potassium salts of fatty acids (insecticidal soap/M-Pede) | Insecticidal soap | Desiccation or membrane disruptor | 12 hours | aphids, caterpillars, fungus gnat adults, leafhoppers, mealybugs, scales, spider mites, whiteflies |
| pyrethrin (Pyganic) | Botanical | Sodium channel blocker [3] | 12 hours | aphids, caterpillars, beetles, mealybugs, thrips, whiteflies |
| spinosad (Entrust) | Spinosyn | Nicotinic acetylcholine receptor agonist and GABA chloride channel activator [5] | 4 hours | caterpillars, thrips, leafminers |
Numbers and letters in brackets [xx] indicate the IRAC (Insecticide Resistance Action Committee) mode of action designation found on the label.
More information about the National Organic Program, online at http://usda.gov/wps/portal/!ut/_s.7_0_A/7_0_1OB?navid=ORGANIC_CERTIFICATIO&navtype=RT&parentnav=AGRICULTURE(opens in new window)
(opens in new window)Biological control suppliers
Sources of biological control agents are listed in the publication Suppliers of Beneficial Organisms in North America by Charles Hunter, which is available from the California Environmental Protection Agency (CEPA) online at http://cdpr.ca.gov/docs/pestmgt/ipminov/bensuppl.htm(opens in new window) or from reputable suppliers.Be sure to consult your biological control supplier to determine the availability and shipping requirements for the natural enemy species you are interested in.
- Green Spot
93 Priest Road
Nottingham, N.H. 03290-6204
603-942-8925
greenmethods.com(opens in new window)
info@greenmethods.com(opens in new window) - IPM Laboratories
P.O. Box 300
Locke, N.Y. 13092-0300
315-497-2063
ipmlabs.com(opens in new window)
ipminfo@Ipmlabs.com(opens in new window) - Koppert Inc.
Romulus, Mich.
734-641-3763
info@koppertline.com(opens in new window) - Syngenta Bioline
Oxnard, Calif.
805-986-8255
info@syngentabioline.com(opens in new window) - BioBest Biological Systems
biobest.be(opens in new window)
info@biobest.ca(opens in new window)
Common greenhouse and nursery pests and pesticides registered for their control. (Always read the label to determine if a pesticide can be used in a particular facility and on a specific crop.)
| Pest | Common name | Trade name |
|---|
| aphids | acephate | Orthene/Precise |
| acetamiprid | TriStar | |
| azadirachtin | Azatin/Ornazin | |
| Beauveria bassiana | BotaniGard | |
| bifenthrin | Talstar/Attain | |
| chlorpyrifos | Duraguard | |
| cyfluthrin | Decathalon/Tempo | |
| dinotefuran | Safari | |
| fenoxycarb | Preclude | |
| fenpropathrin | Tame | |
| flonicamid | Aria | |
| fluvalinate | Mavrik | |
| imidacloprid | Marathon/Merit | |
| kinoprene | Enstar II | |
| methiocarb | Mesurol | |
| neem oil extract | Triact | |
| paraffinic oil | Ultra-Fine oil | |
| petroleum oil | PureSpray Green | |
| potassium salts of fatty acids | Insecticidal soap/M-Pede | |
| pymetrozine | Endeavor | |
| pyrethrin | Pyganic | |
| pyrethrin and silicon dioxide | Diatect V | |
| thiamethoxam | Flagship | |
| beetles | pyrethrin | Pyganic |
| caterpillars | azadirachtin | Azatin/Ornazin |
| Bt spp. kurstaki | Dipel | |
| bifenthrin | Talstar/Attain | |
| cyfluthrin | Decathalon/Tempo | |
| fenoxycarb | Preclude | |
| fenpropathrin | Tame | |
| fenpyroximate | Akari | |
| fluvalinate | Mavrik | |
| novaluron | Pedestal | |
| potassium salts of fatty acids | Insecticidal soap/M-Pede | |
| pyrethrin | Pyganic | |
| pyrethrin and silicon dioxide | Diatect V | |
| spinosad | Conserve/Entrust | |
| fungus gnat | ||
| adult | bifenthrin | Talstar/Attain |
| cyfluthrin | Decathalon/Tempo | |
| fenpropathrin | Tame | |
| fluvalinate | Mavrik | |
| potassium salts of fatty acids | Insecticidal soap/M-Pede | |
| larvae | azadirachtin | Azatin/Ornazin |
| Bt spp. israelensis | Gnatrol | |
| chlorfenapyr | Pylon | |
| chlorpyrifos | Duragard | |
| cyromazine | Citation | |
| diflubenzuron | Adept | |
| kinoprene | Enstar II | |
| pyriproxyfen | Distance | |
| Steinernema feltiae | Nemasys | |
| leaf hoppers | bifenthrin | Talstar/Attain |
| buprofezin | Talus | |
| chlorpyrifos | Duraguard | |
| dinotefuran | Safari | |
| fluvalinate | Mavrik | |
| potassium salts of fatty acids | Insecticidal soap/M-Pede | |
| leaf miners | abamectin | Avid |
| cyromazine | Citation | |
| dinotefuran | Safari | |
| fenoxycarb | Preclude | |
| spinosad | Conserve/Entrust | |
| mealybugs | acephate | Orthene/Precise |
| acetamiprid | TriStar | |
| Beauveria bassiana | BotaniGard | |
| bifenthrin | Talstar/Attain | |
| chlorpyrifos | Duraguard | |
| cyfluthrin | Decathalon/Tempo | |
| fenoxycarb | Preclude | |
| fenpropathrin | Tame | |
| imidacloprid | Marathon/Merit | |
| kinoprene | Enstar II | |
| paraffinic oil | Ultra-Fine oil | |
| petroleum oil | PureSpray Green | |
| potassium salts of fatty acids | Insecticidal soap/M-Pede | |
| pyrethrin | Pyganic | |
| thiamethoxam | Flagship | |
| mites | ||
| broad mite | chlorfenapyr | Pylon |
| spiromesifen | Judo | |
| cyclamen mite | chlorfenapyr | Pylon |
| spider mite | abamectin | Avid |
| acequinocyl | Shuttle | |
| bifenazate | Floramite | |
| chlorfenapyr | Pylon | |
| clofentezine | Ovation | |
| etoxazole | TetraSan | |
| fenbutatin-oxide | ProMite | |
| fenpyroximate | Akari | |
| hexythiazox | Hexagon | |
| milbemectin | Ultraflora | |
| neem oil extract | Triact | |
| paraffinic oil | Ultra-Fine oil | |
| petroleum oil | PureSpray Green | |
| potassium salts of fatty acids | Insecticidal soap/M-Pede | |
| pyridaben | Sanmite | |
| spiromesifen | Judo | |
| plant bugs | bifenthrin | Talstar/Attain |
| fluvalinate | Mavrik | |
| scales | acephate | Orthene/Precise |
| acetamiprid | TriStar | |
| bifenthrin | Talstar/Attain | |
| neem oil extract | Triact | |
| cyfluthrin | Decathalon/Tempo | |
| fenoxycarb | Preclude | |
| imidacloprid | Marathon/Merit | |
| kinoprene | Enstar II | |
| paraffinic oil | Ultra-Fine oil | |
| petroleum oil | PureSpray Green | |
| potassium salts of fatty acids | Insecticidal soap/M-Pede | |
| pyriproxfen | Distance | |
| thiamethoxam | Flagship | |
| shore fly larvae | cyromazine | Citation |
| diflubenzuron | Adept | |
| pyriproxfen | Distance | |
| slugs/snails | methiocarb | Mesurol |
| thrips | abamectin | Avid |
| acephate | Orthene/Precise | |
| azadirachtin | Azatin/Ornazin | |
| bifenthrin | Talstar/Attain | |
| chlorfenapyr | Pylon | |
| chlorpyrifos | Duraguard | |
| cyfluthrin | Decathalon/Tempo | |
| fenoxycarb | Preclude | |
| flonicamid | Aria | |
| fluvalinate | Mavrik | |
| kinoprene | Enstar II | |
| methiocarb | Mesurol | |
| novaluron | Pedestal | |
| pyrethrin | Pyganic | |
| spinosad | Conserve/Entrust | |
| whiteflies | acephate | Orthene/Precise |
| acetamiprid | TriStar | |
| azadirachtin | Azatin/Ornazin | |
| Beauveria bassiana | BotaniGard | |
| bifenthrin | Talstar/Attain | |
| neem oil extract | Triact | |
| cyfluthrin | Decathalon/Tempo | |
| fenoxycarb | Preclude | |
| fenpropathrin | Tame | |
| flonicamid | Aria | |
| fluvalinate | Mavrik | |
| imidacloprid | Marathon/Merit | |
| kinoprene | Enstar II | |
| novaluron | Pedestal | |
| paraffinic oil | Ultra-Fine oil | |
| petroleum oil | PureSpray Green | |
| potassium salts of fatty acids | Insecticidal soap/M-Pede | |
| pymetrozine | Endeavor | |
| pyridaben | Sanmite | |
| pyriproxfen | Distance | |
| pyrethrin | Pyganic | |
| pyrethrin and silicon dioxide | Diatect V | |
| spiromesifen | Judo | |
| thiamethoxam | Flagship |
(opens in new window)Further information
- Albajes, R., M. L. Gullino, J. C. van Lenteren, and Y. Elad (eds.). 1999. Integrated pest and disease management in greenhouse crops. Kluwer Academic Publishers, Netherlands.
- Bennett, K. C (ed.). 2009. Pest management guide for the production and maintenance of herbaceous perennials. Cornell University, Cooperative Extension, Ithaca, N.Y.
- Cloyd, R. A. 2007. Plant protection: Managing greenhouse insect and mite pests. Ball Publishing, Batavia, Ill.
- Dreistadt, S. H. 2001. Integrated pest management for floriculture and nurseries. University of California, Statewide Integrated Pest Management Project, Division of Agriculture and Natural Resources, Publication 3402. Oakland, Calif.
- Gill, S., R. A. Cloyd, J. R. Baker, D. L. Clement, and E. Dutky. 2006. Pests and diseases of herbaceous perennials: The biological approach. Ball Publishing, Batavia, Ill.
- Gill, S., and J. Sanderson. 1998. Ball identification guide to greenhouse pests and beneficials. Ball Publishing, Batavia, Ill.
- Heinz, K. M., R. G. Van Driesche, and M. P. Parrella (eds.). 2004. Biocontrol in protected culture. Ball Publishing, Batavia, Ill.
- Helyer, N., K. Brown, and N. D. Cattlin. 2003. A color handbook of biological control in plant protection. Timber Press, Portland, Ore.
- Hofer, S. E., and D. H. Headrick. 2001. The bug cards: Greenhouse beneficials. Ball Publishing, Batavia, Ill.
- Krischik, V., and J. Davidson (eds.). 2004. IPM (integrated pest management) of Midwest landscapes. Cooperative Project of NCR-193, North Central Committee on Landscape IPM, Minnesota Agricultural Experiment Station SB-07645.
- Lindquist, R. K., and R. A. Cloyd. 2005. Identification of insects and related pests of horticultural plants. Ohio Floriculture Association Services, Inc., Columbus, Ohio.
- Rice Mahr, S. E., R. A. Cloyd, D. L. Mahr, and C. S. Sadof. 2001. Biological control of insects and other pests of greenhouse crops. North Central Regional Publication 581. Cooperative Extension of the University of Wisconsin, Madison, Wis.
- Thomas, C. 2005. Greenhouse IPM with an emphasis on biocontrols. Publication AGRS-96. Pennsylvania Integrated Pest Management Program, Pennsylvania Department of Agriculture, Pennsylvania State University, University Park, Pa.
Soil Steaming to Reduce the Incidence of Soil-borne Diseases, Weeds and Insect Pests
Published: November 24, 2020
Soil steaming is regaining popularity to control weeds, soil-borne diseases and insect pests in agriculture. It is an effective and somewhat sustainable alternative (the drawback is the use of fossil fuel) to chemicals and fumigants to disinfect soil in greenhouses, high tunnels and open fields, and therefore useful in conventional and organic production systems. Hot steam heats up the substrate to temperatures that kill or inactivate weed seeds, nematodes, fungi, bacteria, and viruses by destroying cell structure and proteins. Steaming can also disinfect compost, potting soil, pots, tools, etc.
Frank in Germany developed soil steaming for agricultural purposes in 1888. In the U.S., however, agricultural steamers were first commercialized in 1893 and many steamer designs were developed to disinfect soils in greenhouses and nursery fields. Among the steam application tools were steam rakes, and tractor-drawn steam blades for small acres of high value crops. However, chemicals (pesticides) and fumigants such as methyl-bromide got on the market in the 1950's replacing soil steaming in soil pest management. Nowadays and since the phasing out of methyl-bromide after the Montreal Protocol in 1987, steaming is regaining popularity to disinfecting soil to manage soil-borne diseases, weeds and other pests.
Soil steaming is the transfer of energy from burning fuel through water steam to heat up the soil or substrate to pasteurization or sterilization temperatures. Steam temperature at low pressure is above 212°F, but steam releases large amount of energy when condensates into water heating up the soil with minimal moisture. Soil pasteurization occurs at 160-182°F, but soil sterilization is at the water boiling temperature (212°F). Soil steaming in agriculture is considered a pasteurization process since temperature recommendations are 160°F for 30 minutes to kill most pathogenic fungi, bacteria, insects and nematodes, and 182°F for 30 minutes to kill resistant weed seeds. However, time and energy are necessary to reach those temperatures at the desired soil depth.

Low-pressure steamer (By P. Byers)
Soil revitalization with beneficial microorganisms (soil activator, compost, etc.) may be necessary after soil steaming. When heating to the highest temperatures for sterilization deep into the soil, practically all organisms die, including beneficial ones. Killing soil microorganisms diminishes if not eliminates the soil biological activity, which will affect soil health and nutrient cycling and availability for subsequent crops. In addition, steam killed microorganisms release significant amount of nutrients that were tied up in the living phase of the soil. Therefore, an increase in nutrient availability may enhance vegetative growth in the first crop after treatment, but may require additional amendments or fertilizer for subsequent crops. Consequently, reintroduction of soil beneficial organisms becomes necessary to reactivate and/or maintain the soil biological processes for a healthy and productive soil. Use of quality compost or other type of soil activator will reintroduce beneficial organisms. In the case of compost, plant material infected with soil-borne diseases should not be added and adequate composting temperature must be reached. Otherwise, soil-borne diseases will be reintroduced into the steamed soil because pathogens have resistant structures that will survive organic matter decomposition if killing temperatures are not reached.
There are practically three low pressure steam application types: surface steaming, deep soil steaming and container/stack steaming. There are also several variations within each type as well as applicator systems/designs. Examples are the area sheet steaming, steaming hood, steaming harrow, steaming plough, steam injectors with vacuums, and others.

Surface steaming (By C. Millsap)
Soil surface steaming or sheet steaming is the most economical system and used in high tunnels, greenhouses and field. Steam is injected through a perforated pipe or hose of adequate material laid on the soil surface, so covering the area is necessary to force soil penetration. This steaming type is effective to treat the top 2 to 6 inches of the soil. It is a shallow treatment and the effective treatment depth depends on the time the steaming application last. The longer the application time, the deeper the soil reaches the recommended treatment temperature. Because of the shallow heat treatment, beds/rows should be prepared before steaming and maintained undisturbed after treatment to avoid bringing up pests from soil at depths where killing temperatures were not reached.
Deep soil steaming is more effective and last longer, several years, because it reaches soil depth of 1 to 1.5ft. It reduces the risk of bringing up pests from soil at depths where temperatures were not high enough for killing when working the ground. However, it is more expensive because the increase in energy consumption and treatment time. There are many variations to this technique, but the main ones are depth steaming with vacuum (negative pressure) and the combination of surface and depth steaming (sandwich) system. In the depth steaming with vacuum, surface applied steam is suctioned via pipes installed at the specified depth for this purpose. This is a permanent installation, so the initial cost is significant. Drainage pipes are also used for this purpose. In contrast, mobile systems inject steam at the desire depth and the vacuum pipes are on the surface covered with a hood of aluminum or other corrosion resistant material. The sandwich steaming system, developed in Europe, uses specialized equipment because of the high steam pressure and the necessary hood to force soil penetration. It is more efficient because steam simultaneously penetrates the soil from both the surface and at a specified depth, so it reduces the application time and the total fuel used.
Container/stack steaming is used for potting soil, compost, pots, tools, etc. The use of large containers, boxes, piles, or even dump trailers depends on the amount of substrate. High pressure chambers are also available for specific sterilization purposes. In addition, steam injection via manifolds and using suction systems can heat up large amounts of substrate more evenly. Steamed compost reduces the risk of reintroducing soil-borne diseases back into disinfected soil, but soil revitalization with beneficial microorganisms may be necessary.
Cost of steaming depends on equipment (capacity), area and soil depth to treat, which determine time and fuel needed. Many steamer designs are available, so renting cost may vary depending on the model. However, a low-pressure steamer designed to treat a 300sqft section down to 2inch deep would take approximately 2.5h and 10gal of Diesel. Finally, make sure to monitor temperature when steaming to confirm appropriate disinfection throughout the treated area.
Legalcdn
Well-known member
I like the natural way of removing insects. I am in southwest ontario and recently found out a local company 20 minutes away sells predatory pests.
 www.growliv.com
www.growliv.com
I am not affiliated with them. Just sharing.
Collections
Providing beneficial insects for natural control of pests in greenhouse and field crops.
I am not affiliated with them. Just sharing.
If a person thinks that boron is lacking, how much borax should be used?
Plants must obtain the following mineral nutrients from their growing medium:[2]
- the macronutrients: nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), sulfur (S), magnesium (Mg), carbon (C), hydrogen (H), oxygen (O)
- the micronutrients (or trace minerals): iron (Fe), boron (B), chlorine (Cl), manganese (Mn), zinc (Zn), copper (Cu), molybdenum (Mo), nickel (Ni)
Last edited:
Last edited:
A mosaic virus is any virus that causes infected plant foliage to have a mottled appearance. Such viruses come from a variety of unrelated lineages and consequently there is no taxon that unites all mosaic viruses.
Cleaning and Sanitizing Commercial Greenhouse Surfaces
Posted on May 9, 2023
Sanitizing surfaces is a critical step for greenhouse and nursery sanitation. This is particularly important for sites where disease was a problem in previous crops. Pathogens that cause plant disease can survive in debris, in soil, or on surfaces. Cleaning and sanitizing greenhouses, benches, containers, and tools are important steps in eliminating microscopic propagules that can cause disease in subsequent crops.
Greenhouses and nursery pads should be fallowed (emptied) after each crop cycle or at least once per year for cleaning and sanitizing. Begin by removing weeds, carryover crops, and other potential reservoir hosts. Next, clean and sanitize all surfaces (greenhouse sidewalls, floors, and benches; containers; tools; equipment; irrigation lines) in order to inactivate any remaining propagules.
Cleaning Steps

Steps to cleaning containers, equipment, and surfaces include the following:- Sweep or brush floors and surfaces to eliminate dry debris and soil particles.
- Wash surfaces. Use a brush and heavy stream of water to dislodge large particles. Follow up with soap or detergent. Tools, containers, benches, and equipment should be brushed or rubbed to clean surfaces. Note: Organic regulations indicate that soaps cannot come into contact with food products.
- Rinse away detergent and debris. Repeat steps 1 to 3 until all surfaces are clean.
- Sanitize surfaces, especially if disease was a problem for previous crops. The key to effective disinfection is the length of time the product contacts surfaces; slow-drying increases contact time and optimizes effectiveness. Commercial products are formulated and tested for stability, residual activity, safety, and sensitivity. Refer to label for specific instructions.
- Flush irrigation lines with disinfectant to remove propagules that may have moved into water lines and emitters.
Sanitizers and Disinfectants
Commercial products are recommended for efficacy. The following is a summary, only; refer to product labels for detailed instructions. Mention of trade names is solely for the purpose of providing examples and does not imply endorsement.- Hydrogen dioxide (ZeroTol® 2.0, Oxidate® 2.0) – effective against algae, bacteria, and fungi; contact time 1 to 10 minutes; use on containers, greenhouse walls and floors, foot baths, tools; use with a foaming agent for vertical surfaces (OMRI listed).
- Hydrogen peroxide & peroxyacetic acid (Sanidate 5.0®) – effective against algae, bacteria, and fungi; contact time 1 to 10 minutes; use on containers, greenhouse walls and floors, foot baths, tools; use with a foaming agent for vertical surfaces (OMRI listed).
- Quaternary ammonium compounds (Green-Shield®, Physan 20®, and KleenGrow™) -effective against algae, bacterial, fungi, viruses; contact time 10 to 15 minutes; residual activity several hours; use on containers, cooling pads, greenhouse walls and floors, foot baths, irrigation lines, tools; foaming formulation (OMRI allowed if measures are taken to assure that residues do not come into contact with the fruit or harvestable tissues).
- Chlorine bleach (10% to 20% dilution) – extremely effective against algae, bacteria, fungi, and viruses; contact time less than 1 minute to 15 minutes; most effective product for use on porous surfaces such as wood, especially at higher concentrations; highly corrosive to metals; damaging to soft plastics and rubbers; dangerous to human health; never mix bleach with products containing ammonia or acidic products; half-life 2 hours (OMRI allowed in certain circumstances).
- Alcohol (70%) – practical for use on tools; contact time 10 to 15 minutes; flammable (OMRI allowed for disinfestation of tools).
- Trisodium phosphate, TSP (10% solution) – corrosive to metals; harmful to human health (not allowed by OMRI).
- Lysol® Disinfectant, concentrate – practical for containers, tools, equipment, and for hand-washing smaller surfaces (not allowed by OMRI).
Additional Resources
- Greenhouse Sanitation (PPFS-GH-04)
- OMRI Regulations (link)
Last edited:
Last edited:
Virus Elimination using Microshoot tip therapy
Virus, viroid and virus-like diseases are some of the most important production constraints in vegetatively propagated crops such as berries, citrus, fruit trees, grapes, hops, roses and sweetpotatoes. Plants may become infected with these pathogens by poor propagation practices or in the field. Once a plant is infected with viruses, viroids or virus-like diseases, most cuttings taken from the plant carry them as well. Several types of therapies are used to eliminate viruses and viroids from a plant. They include: microshoot tip culture, meristem culture, embryogenic culture and micrografting. These therapies may also be combined with heat, cryogenic or chemotherapy. Microshoot tip therapy is one of the most reliable methods and has been used for decades on a wide range of ornamental and crop plants.What is a microshoot tip?
A microshoot tip consists of the apical meristem, a dome shaped area at the growing tip of a shoot that contains a few hundred undifferentiated cells, and 2 to 3 pairs of leaf primordia. A microshoot tip is 0.2 to 0.5 mm in size.What is microshoot tip therapy?
Microshoot tip therapy is the process of culturing microshoot tips from an infected plant to generate a population of new plants using tissue culture techniques. The new plants are established in a greenhouse and extensively tested for viruses/viroids. If no pathogens are detected in a plant, it is used as a source of clean propagation material. How is microshoot tip therapy done? Shoot tips that are about 2 to 3 cm long from a vigorously growing plant are harvested into a humid box and taken into the lab. The microshoot tip is excised in sterile conditions using a microscope in a laminar airflow hood. To accomplish this, outer leaves are carefully removed until the meristem dome is exposed. The dome and a few leaf primordia are excised and placed on growth media, or, in the case of citrus, the stem of a nurse plant already growing in a tube.How does microshoot tip therapy work?
It is not known exactly how microshoot tip therapy works but there are several plausible theories. One theory is that the virus has not yet infected the cells in the growing tip because the plant cells are dividing faster than the virus can replicate and infect. In other words, the plant is growing faster than the virus. Another theory is that, by chance, the virus has not infected the microshoot tip. Often viruses are unevenly distributed in a plant and some buds are uninfected. If an uninfected bud is selected, the resulting plant will be clean. Perhaps it works because there is no vascular connection between the youngest part of the tip and the rest of the plant, so the virus is unable to reach the cells at the tip. This is likely the case for phloem-limited viruses (see figure below). There is also evidence that gene silencing mechanisms may be involved in excluding viruses from the meristematic area.How successful is microshoot tip therapy?
Overall microshoot tip therapy treatment success is often over 85%, but it varies with crop species, cultivar and virus. Some crop species and cultivars are recalcitrant to tissue culture; and once in tissue culture, some viruses are difficult to eliminate. Viruses that move readily from cell-to-cell can be very challenging to eliminate. For example, to eliminate raspberry bushy dwarf virus in Rubus spp. a combination of therapies and/or many repetitions are needed. Grapes take an average of 7 months to grow from a less than 0.5 mm microshoot tip to a 6.0 cm plantlet. If the resulting plant is infected, the process is repeated. In berries, each cycle can take 1 to 3 years. Repeated testing after treatment is always necessary to ascertain whether the treatment successfully eliminated the virus.Does microshoot tip therapy lead to unintended effects?
Microshoot tip therapy for virus elimination has been used successfully since the 1960s in many crops with no off-type development or other unintended effects. This is because conditions that may lead to negative effects, such as hormones in growth media and time in culture, are minimized. In addition, since the apical bud is excised along with primordial leaf tissue, cell differentiation has already begun and callus cell development does not occur. Microshoot tip therapy is often confused with tissue culture propagation. Tissue culture propagation involves using larger pieces of tissue, mass production, many subcultures and often many years in culture, which can lead to undesirable traits in plant progeny. Finally, tissue culture propagation does not eliminate viruses. Short videos showing the excision process under the scope for several crops are available at the Foundation Plant Services, UC Davis youtube channel.
Surprisingly, a high percentage of seed transmission for HLVd was detected in our current study. Transmission percentages of 58 to 84% were observed when an infected male parent or infected female parent was used, respectively. Future studies will be performed to investigate the potential for pollen and seed transmission of HLVd across host species and to develop methods for screening hemp seeds to segregate and certify healthy seeds.
To date, HLVd-resistant cannabis cultivars are not known. Meristem tissue culture is the only effective control method via which infected plants can be saved. This process is both laborious and expensive. Although tissue-cultured plantlets are viroid-free, it is important to understand that they are not viroid-resistant. Hence, following preventive measures in order to avoid an insurge of HLVd into a given growth environment is crucial in HLVd-associated disease management. However, for sustainability reasons, it is important to find a practical long-term solution by employing strategies such as the control of HLVd infection by breeding HLVd-resistant plants, by cross-protection and by developing RNA interference-mediated resistance in the plants.
New Insights into Hop Latent Viroid Detection, Infectivity, Host Range, and Transmission
Department of Plant Pathology & Microbiology, Texas A&M University, College Station, TX 77843, USAAuthor to whom correspondence should be addressed.
Viruses 2024, 16(1), 30; https://doi.org/10.3390/v16010030
Submission received: 30 October 2023 / Revised: 21 December 2023 / Accepted: 21 December 2023 / Published: 23 December 2023
Last edited:
Hop Latent Viroid (HLVd) & Pathogen Diagnostics: A Comprehensive Overview - Cannabis Industry Journal
Hop Latent Viroid (HLVd) threatens cannabis crops and results in reduced yield, quality and profit. However, the lack of education and research about this pathogen poses a bigger threat to the cannabis community. To safeguard the productivity and quality of cannabis crops we must learn accurate...
July 5, 2023
Hop Latent Viroid (HLVd) & Pathogen Diagnostics: A Comprehensive Overview
By Tassa Saldi, Ph.D. No Comments

Hop Latent Viroid (HLVd) threatens cannabis crops and results in reduced yield, quality and profit. However, the lack of education and research about this pathogen poses a bigger threat to the cannabis community. To safeguard the productivity and quality of cannabis crops we must learn accurate and timely pathogen identification along with proactive disease management practices.
Hop latent viroid (HLVd) has gained attention as the molecular cause of “dudding disease” and is causing significant economic losses in the cannabis industry.1,2 Estimates indicate that upwards of 4 billion dollars of market value are lost each year to this pathogen alone.3 The impact of HLVd on cannabis plants necessitates the development and implementation of effective pathogen diagnostics to mitigate its spread and minimize crop damage. With collaborative research efforts, we can gain valuable insights into the characteristics, spread, symptoms and preventive measures associated with HLVd in the cannabis industry.
Viroids: A Brief Overview

Figure 1: Virus vs Viroid
Viroids are unique infectious agents composed solely of genetic material, distinct from viruses. Unlike viruses, viroids lack a protective protein layer and solely rely on the host plant for replication and spread. Their stability and ability to persist in various environments make viroids a formidable threat to plant health.
Hop Latent Viroid: Origin and Global Spread
Hop latent viroid was initially identified in hop plants in 19884 and was found to be largely asymptomatic in this crop. Consequently, HLVd has spread worldwide, mostly unchecked by the hops industry. This pathogen has been identified on most continents and in some fields more than 90% of hops plants are infected.5 Hop latent viroid very likely jumped from hops into cannabis, due to similar genetics. The timing and mechanism of cross-species transmission to cannabis remains unknown, but the prevalence of HLVd suggests this viroid has been circulating within cannabis for an extended period. Data collected at TUMI Genomics indicates that HLVd is present in all states in the United States where cannabis is legal as well internationally including; Canada, the United Kingdom, France, the Netherlands, Thailand, Austria and Switzerland.Symptoms and Impacts on Cannabis Plants

Figure 2: HLVd Symptoms
HLVd exhibits a wide range of symptoms, which can vary from severe to subtle, affecting the growth, leaf development, flower quality and overall vitality of cannabis plants. Understanding these symptoms is crucial for timely diagnosis and appropriate disease management strategies. However, HLVd can also present asymptomatically, especially in vegetative plants. The only way to determine if your plants are infected is by routine molecular testing.
Modes of Transmission
Mechanical Transmission: HLVd primarily spreads mechanically through contact with infected sap during activities like trimming and handling. Additionally, transmission through contaminated water and the potential role of insects, fungal pathogens and seeds in spreading HLVd have also been observed.Seed Transmission: Although no published studies exist in cannabis describing the frequency of seed transmission, HLVd does transmit through seeds in hop plants at a rate of around 8%.7 Preliminary studies performed by TUMI Genomics in collaboration with EZ-genetics suggest cannabis seed transmission does occur at variable rates depending on strain and level of infection of the parent plants.
Water Transmission: It has also been observed that viroids are in high concentration in the roots8 and can move from the root into runoff water.9 Plants sharing a common water source with infected plants, such as recirculating water systems or flood and drain procedures, are at risk for transmission of the viroid.
Insect and Other Vector Transmission: The jury is still out as to whether or not insects can transmit HLVd. However, multiple viroids are transmitted via insects, so it is likely that HLVd insect transmission occurs. Recent studies also indicate that fungal pathogens, like Fusarium, can transmit viroid infections.6 While pathogenic fungus is a major concern for cannabis growers in its own right, limiting the prevalence and spread of fungal pathogens in your facility could help limit hop latent viroid transmission as well.
Therefore, implementing proper sanitation practices and limiting pest access can help minimize transmission risks.
Preventive Measures
Prevention plays a vital role in safeguarding cannabis crops against HLVd. The STOP program, developed by TUMI Genomics, offers a comprehensive approach that includes maintaining a Sterile environment, Testing mother plants regularly, Organizing the facility to minimize pathogen spread, and Protecting the facility’s borders from introduction of infected plant material, insects and contaminated water. More details on these preventative measures can be found here.Pathogen Diagnostics
Protecting your plants from hop latent viroid requires accurate identification and removal of infected plants before the infection spreads to other plants. To accomplish this, several critical factors should be considered:Type of test: HLVd and all viroids can only be detected by a molecular test (a test that detects the presence of DNA/RNA). Among common molecular tests, PCR is generally the most sensitive and accurate method. PCR can provide both a diagnosis and an approximate viroid level, allowing informed management decisions. Other types of molecular tests, such as LAMP and RPA, can formally be as sensitive as PCR, but the classic versions of these assays often suffer from false positive/negative results, reducing accuracy.
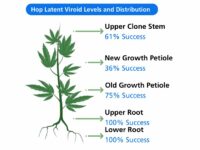
Figure 3: HLVd Levels and Distribution
Tissue type: An important consideration for HLVd detection is the plant tissue selected for testing, especially when identifying low-level or early infections when HLVd is not yet systemic. Studies completed by TUMI Genomics and others show root tissue contains the highest levels of HLVd and is the most reliable tissue for detection of viroid infection. While upper root tissue appears to contain the highest levels of viroid, roots from anywhere in the root ball are predictive of infection. Samples taken from the leaves/foliage tend to have lower levels of viroid and may produce false negative results.
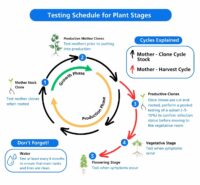
Figure 4: Testing Schedule
Testing frequency: Routine pathogen testing is standard practice in general agriculture and is critical to maintain a healthy cannabis crop. Testing of mother plants every 4-6 weeks for economically critical pathogens (such as HLVd) will help ensure a successful run and a high-quality product.
Disinfection Methods
Studies have shown that viroids can remain infectious for longer than 24 hours on most common surfaces11 and 7 weeks in water.10 Making effective disinfection methods essential to limit the spread of HLVd. While common disinfectants like alcohol and hydrogen peroxide are ineffective against viroids, a 10% bleach solution has shown efficacy in destroying HLVd. Proper tool sterilization practices, such as soaking tools in bleach for 60 seconds, are crucial to prevent transmission during plant handling.
Figure 5: Bleach Dilution
Hop latent viroid poses a significant threat to the cannabis industry, leading to substantial economic losses. Timely and accurate pathogen diagnostics, along with stringent preventive measures, are essential for minimizing the impact of HLVd. Regular testing, proper disinfection protocols and adherence to pathogen prevention programs can help ensure the health and vitality of cannabis crops in the face of this global pandemic.
References
- Bektas, A., et al. “Occurrence of Hop Latent Viroid in Cannabis Sativa with Symptoms of Cannabis Stunting Disease in California.” APS Journals, 21 Aug. 2019, doi.org/10.1094/PDIS-03-19-0459-PDN.
- Warren, J.G., et al. “Occurrence of Hop Latent Viroid Causing Disease in Cannabis Sativa in California.” APS Journals, 21 Aug. 2019, doi.org/10.1094/PDIS-03-19-0530-PDN.
- Cooper, Benjie. “Hop Latent Viroid Causes $4 Billion Cannabis Industry Loss – Candid Chronicle.” Candid Chronicle – Truthful, Straightforward, Blunt Cannabis News, 16 Aug. 2021, candidchronicle.com/hop-latent-viroid-causes-4-billion-cannabis-industry-loss/.
- Puchta H, Ramm K, Sänger HL. The molecular structure of hop latent viroid (HLV), a new viroid occurring worldwide in hops. Nucleic Acids Res. 1988 May 25;16(10):4197-216. doi: 10.1093/nar/16.10.4197. PMID: 2454454; PMCID: PMC336624.
- Faggioli, Franceso, et al. “Geographical Distribution of Viroids in Europe.” Viroids and Satellites, 31 July 2017, www.sciencedirect.com/science/article/abs/pii/B9780128014981000449#bib47.
- Wei S, Bian R, Andika IB, Niu E, Liu Q, Kondo H, Yang L, Zhou H, Pang T, Lian Z, Liu X, Wu Y, Sun L. Symptomatic plant viroid infections in phytopathogenic fungi. Proc Natl Acad Sci U S A. 2019 Jun 25;116(26):13042-13050. doi: 10.1073/pnas.1900762116. Epub 2019 Jun 10. PMID: 31182602; PMCID: PMC6600922.
- Singh RP. The discovery and eradication of potato spindle tuber viroid in Canada. Virus disease. 2014 Dec;25(4):415-24. doi: 10.1007/s13337-014-0225-9. Epub 2014 Dec 2. PMID: 25674616; PMCID: PMC4262315.
- Jama, Aisha, et al. TUMI Genomics, Fort Collins, CO, 2022, Hop Latent Viroid Levels and Distribution in Cannabis Plant Tissue.
- Mackie AE, Coutts BA, Barbetti MJ, Rodoni BC, McKirdy SJ, Jones RAC. Potato spindle tuber viroid: Stability on Common Surfaces and Inactivation With Disinfectants. Plant Dis. 2015 Jun;99(6):770-775. doi: 10.1094/PDIS-09-14-0929-RE. Epub 2015 May 15. PMID: 30699527.
- Mackie AE, Coutts BA, Barbetti MJ, Rodoni BC, McKirdy SJ, Jones RAC. Potato spindle tuber viroid: Stability on Common Surfaces and Inactivation With Disinfectants. Plant Dis. 2015 Jun;99(6):770-775. doi: 10.1094/PDIS-09-14-0929-RE. Epub 2015 May 15. PMID: 30699527.
- Mackie AE, Coutts BA, Barbetti MJ, Rodoni BC, McKirdy SJ, Jones RAC. Potato spindle tuber viroid: Stability on Common Surfaces and Inactivation With Disinfectants. Plant Dis. 2015 Jun;99(6):770-775. doi: 10.1094/PDIS-09-14-0929-RE. Epub 2015 May 15. PMID: 30699527.
About The Author

Tassa Saldi, Ph.D.
Honestly,
number 1 this should be a sticky!
and 2.. acespicoli should be the only one to add to this thread. or
a offshoot thread where questions can be asked based on this thread should be made so it doesnt get clouded by questions. (yes, even like this post) or
a mod come in and clear up posts inbetween aces posts. move them later down. or reserve the first pages for ace only.
I have been reading cannabis related disease articles, studies and or scholarly articles for some time, an this is by far one of the best consolidated threads i have EVER SEEN
In the link below there is a bunch of good articles from the university of ct hemp program

 cannabis.cahnr.uconn.edu
cannabis.cahnr.uconn.edu
Thank you acespicoli....
number 1 this should be a sticky!
and 2.. acespicoli should be the only one to add to this thread. or
a offshoot thread where questions can be asked based on this thread should be made so it doesnt get clouded by questions. (yes, even like this post) or
a mod come in and clear up posts inbetween aces posts. move them later down. or reserve the first pages for ace only.
I have been reading cannabis related disease articles, studies and or scholarly articles for some time, an this is by far one of the best consolidated threads i have EVER SEEN
In the link below there is a bunch of good articles from the university of ct hemp program

Resources | CAHNR Cannabis Programs
Hemp Growers Meeting at Tolland County Extension CenterPresentations from June 26, 2019 Growing Hemp in Controlled Environment, Shelley Durocher-Nesta Growi ...
Thank you acespicoli....

Last edited:
Honestly,
number 1 this should be a sticky!
and 2.. acespicoli should be the only one to add to this thread. or
a offshoot thread where questions can be asked based on this thread should be made so it doesnt get clouded by questions. (yes, even like this post) or
a mod come in and clear up posts inbetween aces posts. move them later down. or reserve the first pages for ace only.
I have been reading cannabis related disease articles, studies and or scholarly articles for a long time, an this is by far one of the best consolidated Threads i have EVER SEEN.
In the link below there is a bunch of good articles from the university of ct hemp program

Resources | CAHNR Cannabis Programs
Hemp Growers Meeting at Tolland County Extension CenterPresentations from June 26, 2019 Growing Hemp in Controlled Environment, Shelley Durocher-Nesta Growi ...cannabis.cahnr.uconn.edu
Thank you acespicoli....
will make the first post a table of contents with links
Very kind, Thank you! Happy New Year






