acespicoli
Well-known member





| Disease | Active compound | Receptors | Mechanism of Action |
|---|---|---|---|
| Parkinson’s | THC, CBD | Non-Cannabinoid receptors | Antioxidant activity |
| CB2 | Decreases proinflammatory cytokines and NFkb pathway activity | ||
| THCV | CB2 | Decreases proinflammatory cytokines & ROS. Increases anti-inflammatory cytokines | |
| Huntington Disease | THC, CBD | Non-Cannabinoid receptors | Decreases oxidative stress and edema |
| THC | CB1 | Neuroprotective activity | |
| CBG | Cannabinoid & Non-cannabinoid receptors | Reduces mutant Htt protein aggregation. Counteract superregulation of proinflammatory markers | |
| Alzheimer’s disease | THC | CB1 | Inhibits AChE neurotransmitter |
| CBD | CB2 | Inhibits amyloid-β plaque formation. Modulates iNOS expression | |
| CBD | PPAR-y | Decreases amyloid-β production and reduces apoptosis | |
| Multiple Sclerosis | THC | CB1 | Reduces neuroinflammation. Prevents excitotoxicity by reducing glutamate release |
| Amyotrophic Lateral Sclerosis | THC | CB2 | Reduces excitotoxicity by reducing glutamate release. Reduces oxidative cell damage. |
| Hypoxia-Ischemia | CBD | CB2, 5HT1A | Modulation of oxidative stress and inflammation. Modulation of glutamate and cytokine release. Modulation of COX-3 induction |
| Epilepsy | CBD | GPR55, TPRV-1, 5HT1A | Reduction of Ca2+ in synaptic complex of neurons |
| THC | CB1 | Modulates GABA release | |
| Pain | THC, CBD | CB1, CB2 | Inhibits the release of neurotransmitters from presynaptic nerve. Activates the descending inhibitory pain pathways. |
| CBD | TRPV-1 | Desensitizes TRPV-1 receptor and inhibits activation of other intermediary molecules of signaling pathway. | |
| Apptetite | THC, CBD | CB1, CB2 | Upregulates ghrelin and downregulates leptin hormone |
| Respiratory Disorder | THC | CB1, CB2 | Prevents fibrosis in lung, liver, heart, kidney, and skin cells. Reduces inflammation, oxidative/nitrosative stress. |
Going to start with this rate to make cannabis capsules. Need to work up to a comfortable dose using this variety.1 gram decarbed Hash
5 ml coconut oil (1 teaspoon)
2ml lecithin (1/4 teaspoon)
Heat 220 F for 10 minutes
Freeze
Reheat.
Fills about 10 capsules. Same general strength as the 5 gram version.
What is the general THC content of kief per gram?
 Depends on the starting material cbd/thc etc, so without a tester or lab work
Depends on the starting material cbd/thc etc, so without a tester or lab work 
This is something I intend to persue in earnest with cbd rich ... well also balanced thc/cbd strains to use as feed material for these medications. Also the potency and tolerance needs to be monitored closely.
RSO FECO Liposomal Encapsulation | badkittysmiles
www.badkatscannapharm.com
Lots of great methods of making concentrates on here and also a number of great cannabis infused recipes.
 Thx to everyone working on this and sharing your experiences
Thx to everyone working on this and sharing your experiences

THE CARBON COPY OF THE SITE IS HERE FOR REFERENCE
RSO FECO Liposomal Encapsulation | badkittysmiles
www.badkatscannapharm.com
Lots of great methods of making concentrates on here and also a number of great cannabis infused recipes.


 added this one too have not tried it, also interested in cbd salves for joint pain topical treatments
added this one too have not tried it, also interested in cbd salves for joint pain topical treatments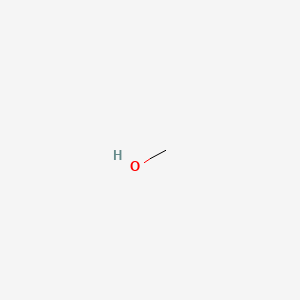



Didnt see this but the above post may be of helpfulWhat is the best method of making a tincture? Some say to shake with alcohol for 10 seconds then strain, some recommend steeping for a couple days in the refrigerator. I guess it depends on the concentration of the starting material, whether its trim from harvest or fully matured flowers. Must be ground up I would assume.
Does mint increase activity or just hide the smell?
How do you determine how to dilute it? I guess take 1 drop at a time and increase calculate the dilution needed.

Thanks or the info my brother has multiple sclerosis.Medical Condition
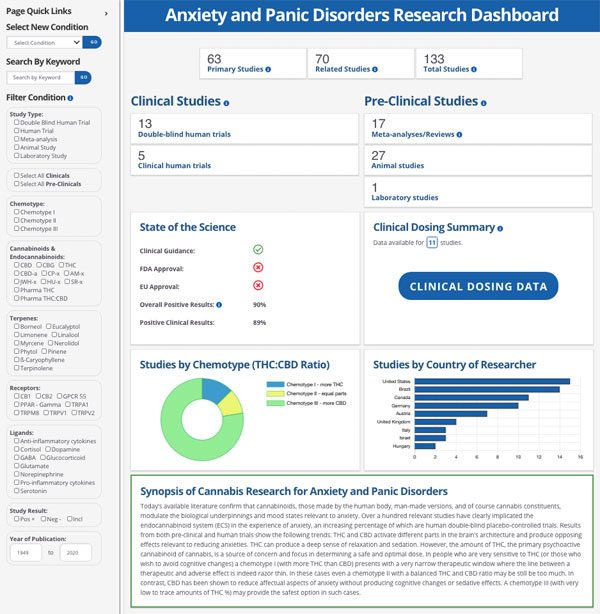
Latest medical research - can cannabis treat your medical condition? Learn the THC:CBD ratios. Try CannaKeys research tools. 1 Wk Free Trial.cannakeys.com
CannaKeys: Unlocking The Science
Medical Condition
The CannaKeys 360° Medical Condition Search is the landing page where you can access those conditions for which there are degrees of scientific evidence that inform us if cannabis is posited to work for a particular disease.
Each condition listed is a live link to its corresponding dashboards. So once the page opens, you’ll have at your fingertips the state of the science and its broader context for you to make more informed and discerning decisions to optimize patient outcomes.

Dashboard & Filter
- Research dashboard presents the total number of studies with study types, the clinical/preclinical breakdown, a strength of science for the condition and cannabis chemotypes, and charts with overview information such as countries where the studies were done and the phyto-cannabinoid chemotypes the research examined.
- Summary of the cannabis research that provides the current status of the efficacy of cannabinoid therapies for the condition searched.
- Dashboard filter allows users to filter the search results by seven different data categories including study type, cannabinoid, and therapeutic result of the study’s findings.

The Studies
- Comprehensive and curated list of studies for the medical condition searched.
- Primary studies that directly examine the condition in question.
- Related studies that explore related symptoms or conditions.
- Filter studies by Type of Study, Therapeutic Effect, and various data points and study components.

Condition Summary
- Summary for the condition including: overview description, symptoms, synonyms for the condition
- Associated ICD-10 codes and ranges based on the databank’s approach to categorizing the health conditions.

Drug Interactions & Dosing
- Drug interactions information for cannabis in general
- Dosing considerations provide a framework for understanding dosing definitions and ranges
Thanks or the info my brother has multiple sclerosis.
Starting with the trichomes is a good way to standardize dosage batch to batch.


 drive.google.com
drive.google.com