Published online 2020 Jun 26. doi: 10.1104/pp.20.00593


 en.wikipedia.org
en.wikipedia.org

 en.wikipedia.org
en.wikipedia.org

 en.wikipedia.org
en.wikipedia.org

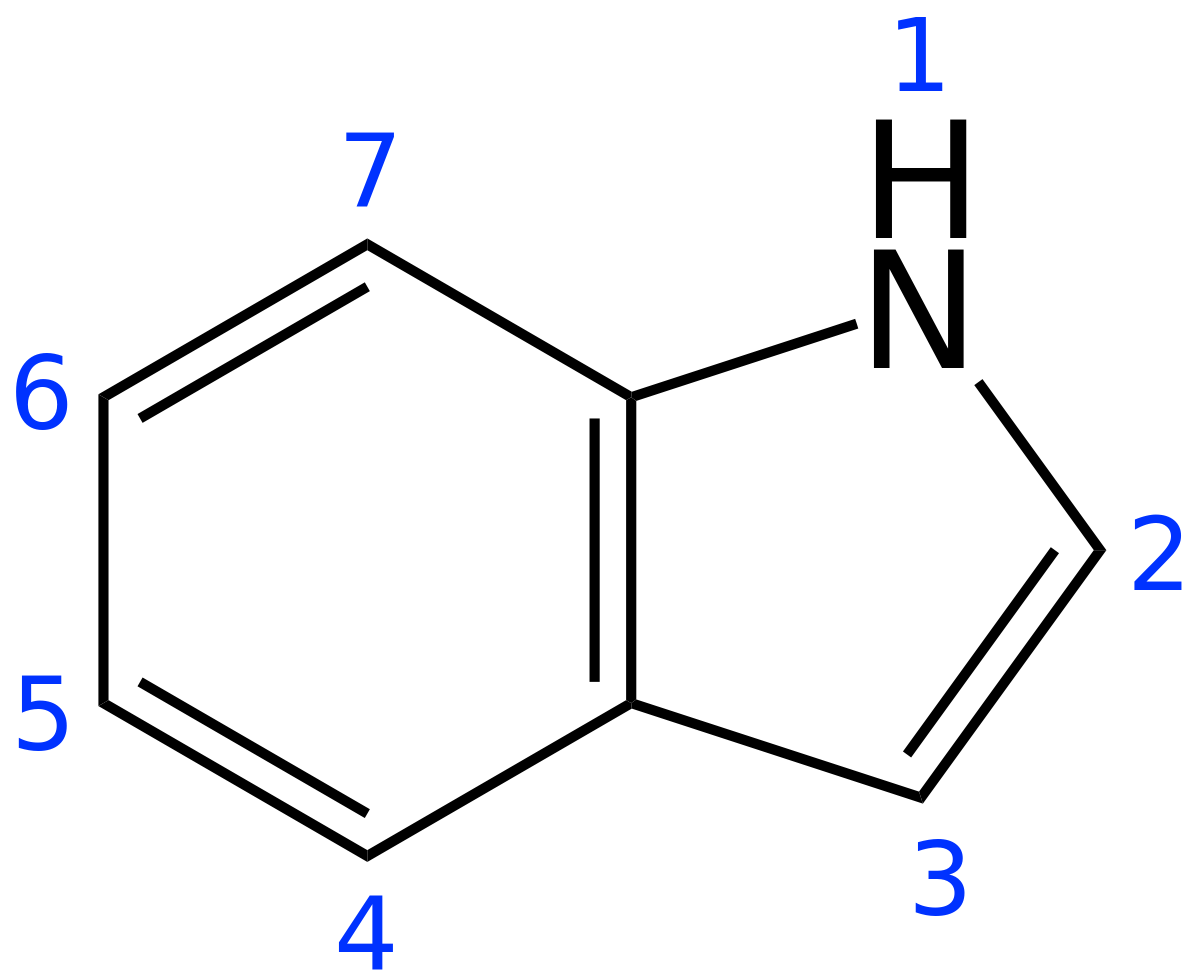
https://en.wikipedia.org/wiki/Muscone
MUSK DEER SKULL
The musk deer belongs to the family Moschidae and lives in Tibet,[8] India, Nepal, Pakistan, Afghanistan, China, Siberia, Mongolia, Manchuria, Korea and North Vietnam. The musk pod, a preputial gland in a pouch, or sac, under the skin of the abdomen of the male musk deer, is normally obtained by killing the male deer through traps laid in the wild. Upon drying, the reddish-brown paste inside the musk pod turns into a black granular material called "musk grain", which is then tinctured with alcohol. The aroma of the tincture gives a pleasant odor only after it is considerably diluted.
No other natural substance has such a complex aroma associated with so many contradictory descriptions; however, it is usually described abstractly as animalistic, earthy and woody[5] or something akin to the odor of baby's skin.[9]
Musk has been a key constituent in many perfumes since its discovery, being held to give a perfume long-lasting power as a fixative. Today, the trade quantity of the natural musk is controlled by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), but illegal poaching and trading continues.[9]

 en.wikipedia.org
The African civet, Civettictis civetta,[1] has historically been the main species from which a musky scent used in perfumery, also referred to as "civet", was obtained.
en.wikipedia.org
The African civet, Civettictis civetta,[1] has historically been the main species from which a musky scent used in perfumery, also referred to as "civet", was obtained.

TERPINOLENE CAT PISS / AMMONIA
CAT PISS / AMMONIA

 en.wikipedia.org
en.wikipedia.org

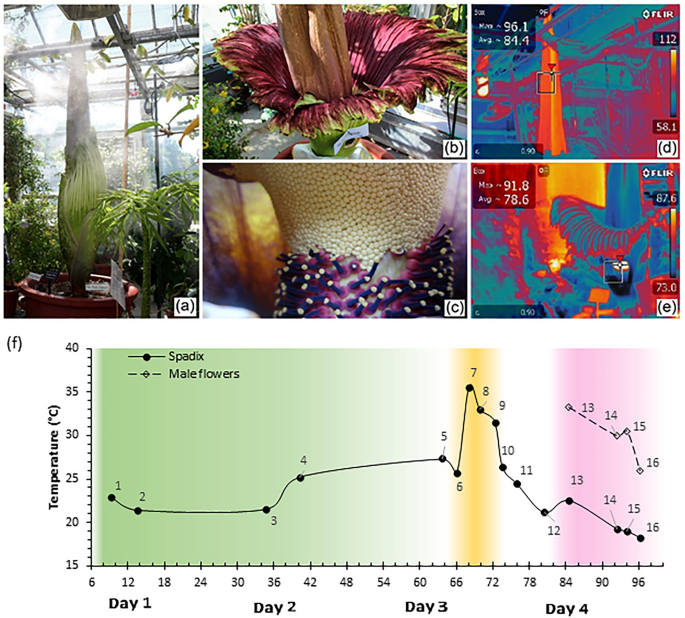
A. titanum is native solely to western Sumatra
How the volatile organic compounds emitted by corpse plant change through flowering. S
ci Rep 13, 372 (2023).
 doi.org
Kang, L., Kaur, J., Winkeler, K. et al.
doi.org
Kang, L., Kaur, J., Winkeler, K. et al.
Download citation
dimethyl trisulfide (foul odor9),
methyl thioacetate (sulfurous odor10),
and isovaleric acid (cheesy, sweaty odor10,11).
^^^LINKED FOR CONVENIENCE^^^
The diffusion of volatile molecules from the flowers is enhanced by thermogenesis. The spadix thermogenesis period starts after the opening of the spathe on the first day, reaching 36 °C, in pulses, synchronizing with the waves of the carrion-like odor12. The thermogenesis of male flowers begins on the second day when pollens are being released, where the temperature of the florets can also reach up to 36 °C13. The flowering A. titanum draws insects that are typically attracted to carrion, including dung beetles and flesh flies9.
https://en.wikipedia.org/wiki/Dracunculus_vulgaris#Cultivation
Common names include the
common dracunculus, dragon lily, dragon arum, black arum and vampire lily.
In Greece, part of its native range, the plant is called drakondia, the long spadix being viewed as a small dragon hiding in the spathe.[2]
This herbaceous perennial is endemic to the Balkans, extending as far as Greece, Crete, and the Aegean Islands, and also to the south-western parts of Anatolia
Eastern skunk cabbage
(Symplocarpus foetidus) is also known as polecat weed, skunk weed, and swamp cabbage.

"Smell", from Allegory of the Senses by Jan Brueghel the Elder, Museo del Prado
Terpene Synthases and Terpene Variation in Cannabis sativa

Rhododendron dauricum - Wikipedia

Cannabichromene - Wikipedia

Cannabis flower essential oil - Wikipedia
https://en.wikipedia.org/wiki/Muscone
MUSK DEER SKULL
The musk deer belongs to the family Moschidae and lives in Tibet,[8] India, Nepal, Pakistan, Afghanistan, China, Siberia, Mongolia, Manchuria, Korea and North Vietnam. The musk pod, a preputial gland in a pouch, or sac, under the skin of the abdomen of the male musk deer, is normally obtained by killing the male deer through traps laid in the wild. Upon drying, the reddish-brown paste inside the musk pod turns into a black granular material called "musk grain", which is then tinctured with alcohol. The aroma of the tincture gives a pleasant odor only after it is considerably diluted.
No other natural substance has such a complex aroma associated with so many contradictory descriptions; however, it is usually described abstractly as animalistic, earthy and woody[5] or something akin to the odor of baby's skin.[9]
Musk has been a key constituent in many perfumes since its discovery, being held to give a perfume long-lasting power as a fixative. Today, the trade quantity of the natural musk is controlled by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), but illegal poaching and trading continues.[9]
-

Moschus moschiferus, Siberian musk deer -

"Musk-cat", woodcut from Hortus Sanitatis, 1491

Civet - Wikipedia
TERPINOLENE
 CAT PISS / AMMONIA
CAT PISS / AMMONIA
Carrion flower - Wikipedia
A. titanum is native solely to western Sumatra
How the volatile organic compounds emitted by corpse plant change through flowering. S
ci Rep 13, 372 (2023).

How the volatile organic compounds emitted by corpse plant change through flowering - Scientific Reports
The corpse plant (Amorphophallus titanum) is so named because it produces a pungent, foul odor when flowering. Little is known about how the emitted volatiles change throughout the two-day flowering period. In this study, the comprehensive monitoring of the presence and change in volatile...
Download citation
- Received17 July 2022
- Accepted26 December 2022
- Published07 January 2023
- DOIhttps://doi.org/10.1038/s41598-022-27108-8
dimethyl trisulfide (foul odor9),
methyl thioacetate (sulfurous odor10),
and isovaleric acid (cheesy, sweaty odor10,11).
^^^LINKED FOR CONVENIENCE^^^
The diffusion of volatile molecules from the flowers is enhanced by thermogenesis. The spadix thermogenesis period starts after the opening of the spathe on the first day, reaching 36 °C, in pulses, synchronizing with the waves of the carrion-like odor12. The thermogenesis of male flowers begins on the second day when pollens are being released, where the temperature of the florets can also reach up to 36 °C13. The flowering A. titanum draws insects that are typically attracted to carrion, including dung beetles and flesh flies9.
https://en.wikipedia.org/wiki/Dracunculus_vulgaris#Cultivation
Common names include the
common dracunculus, dragon lily, dragon arum, black arum and vampire lily.
In Greece, part of its native range, the plant is called drakondia, the long spadix being viewed as a small dragon hiding in the spathe.[2]
This herbaceous perennial is endemic to the Balkans, extending as far as Greece, Crete, and the Aegean Islands, and also to the south-western parts of Anatolia
Eastern skunk cabbage
(Symplocarpus foetidus) is also known as polecat weed, skunk weed, and swamp cabbage.
"Smell", from Allegory of the Senses by Jan Brueghel the Elder, Museo del Prado
Last edited: