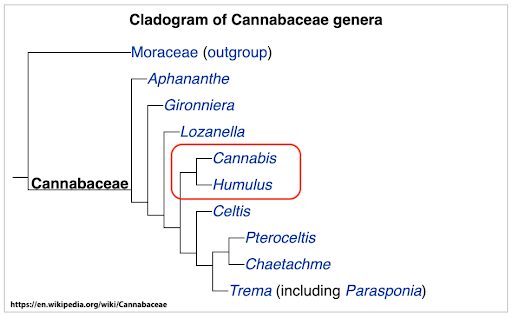
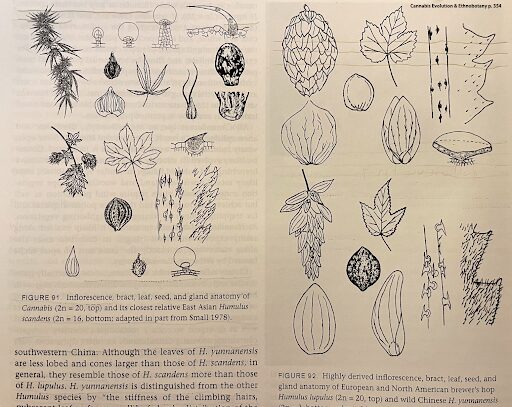
POTENCY
YIELD
DURATION
CHEMO-TYPE COMPLEXITY
FLOWER MATURITY TYPE ALT PHYLLOTAXY
INDOOR VS OUTDOOR MORPHOLOGY
PERFECTLY IMPERFECT
NOVELTY
WILD
YIELD
DURATION
CHEMO-TYPE COMPLEXITY
FLOWER MATURITY TYPE ALT PHYLLOTAXY
INDOOR VS OUTDOOR MORPHOLOGY
PERFECTLY IMPERFECT
NOVELTY
WILD









 Pin It
Pin It