Old Piney
Well-known member
Nice! I'm wondering what haze is this and what latitude are you atOutdoor Haze...
Nice! I'm wondering what haze is this and what latitude are you atOutdoor Haze...
hello friends of the fresh air ;-)
here my outdoor haze this season...
like same than last from size ... around 4 meter + but different genetics...
can't wait for smoke test hehe
well here in north Germany we had 2 weeks cold rainy and stormy weather with 3°C @ night and 10°C @ day... they made it... no mold no other plant issues... only mouse and rat's that eat the roots on some...
now we have a few day's around 20°C to finish and give some THC shot @ the end... after all I'll can show again that sativa rocks solid in harsh condition
52.7 ... north germany ...Nice! I'm wondering what haze is this and what latitude are you at
YESGrown in North Germany?
Wow! Good
Looking for that supper early outdoor pure Sativa? Khalifa’s Moroccan Beldia. She's not all that strong but she's got a lovely floaty high and she's tough as nails outside. One of my personal favorites. Looking forward to a smoke report on your hazeSativa rules for our outdoor grows
View attachment 19088489
The chemistry of roots primarily involves the release of various organic compounds, known as "root exudates,"which significantly impact the soil environment around the root system (rhizosphere), influencing nutrient uptake, microbial interactions, and even communication with neighboring plants; these exudates can include organic acids like citric and malic acid, sugars, amino acids, enzymes, and secondary metabolites like phenolics and terpenes, which can vary depending on the plant species and environmental conditions.
Key aspects of root chemistry:
Important concepts related to root chemistry:
- Root exudates:
These substances secreted by roots play a crucial role in mobilizing nutrients in the soil by altering pH levels and solubilizing minerals like phosphorus.
- pH modification:
Roots can actively acidify the rhizosphere by releasing hydrogen ions, helping to dissolve minerals that are otherwise unavailable.
- Nutrient acquisition:
Specific chemical signals released by roots can attract beneficial soil microbes, like mycorrhizal fungi, which facilitate the uptake of nutrients like phosphorus and nitrogen.
- Allelopathy:
Some plants release chemical compounds through their roots that can inhibit the growth of neighboring plants, creating competition dynamics.
- Chemical diversity:
The composition of root exudates varies based on the plant species, including primary metabolites like sugars and amino acids, and secondary metabolites like flavonoids, alkaloids, and terpenes.
- Rhizosphere:
The area of soil directly surrounding plant roots where most root-soil interactions occur.
- Mycorrhizal associations:
Symbiotic relationships between plant roots and fungi that enhance nutrient uptake, particularly phosphorus.
- Signal molecules:
Specific chemical compounds released by roots that can trigger responses in other organisms, including microbes and neighboring plants.
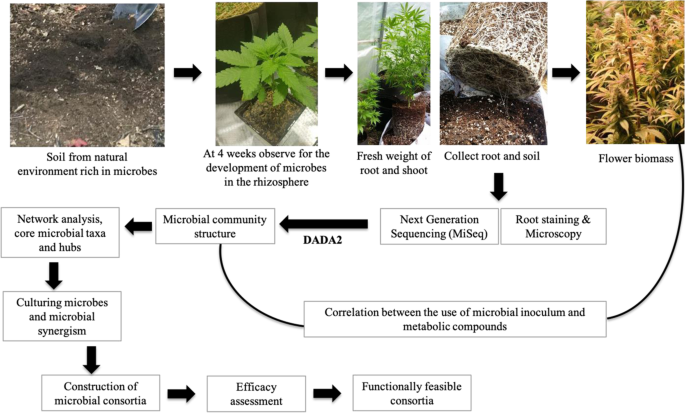
This illustration shows the technical workflow for the development of microbial consortia for Cannabis (hemp and marijuana). The source is a microbial suspension from a natural environment rich in microbes. A root colonization study at 4 weeks allows assessment of whether microbes have been established. Next-generation sequencing and the DADA2 pipeline in R can determine community composition, and core microbes can be isolated from cannabis roots and rhizospheric soils. Network analysis will provide insight into the core microbial taxa and hub microbes. Later, the core microbes can be cultured in different microbial growth mediums and microbial synergism can be evaluated. Then, different microbial consortia can be considered for efficacy assessment, and functionally feasible consortia considered for utilization in the cultivation of marijuana and hemp
Interactions in the Rhizosphere | Image Credit: https://encyclopedia.pub/entry/history/compare_revision/55423/-1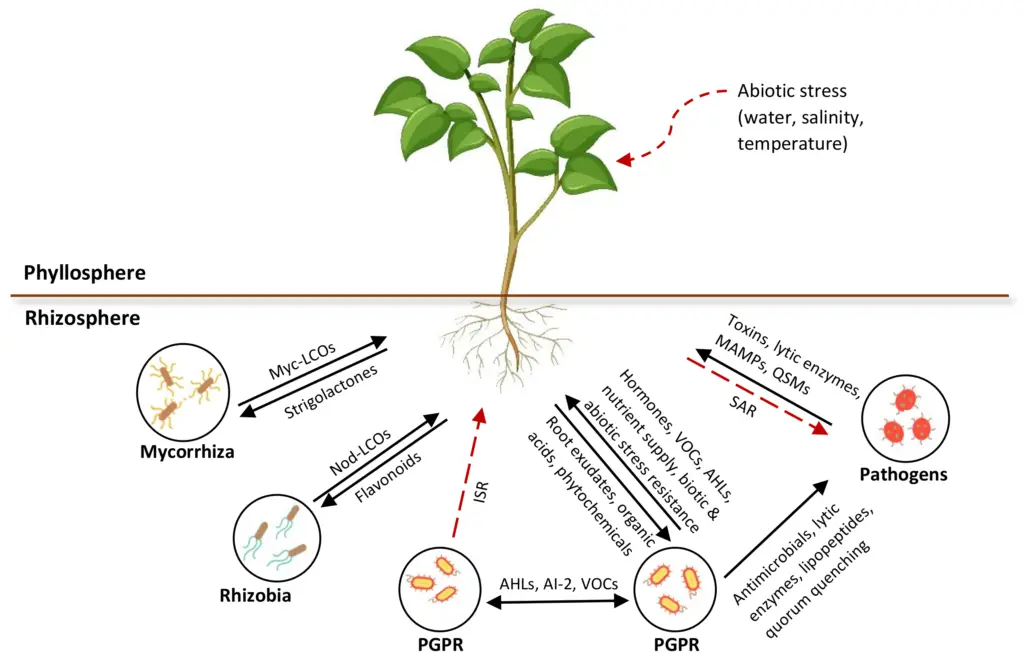
View attachment 19088562
1. Dinitrogen-Fixation Symbiosis
- Dinitrogen-fixing bacteria are able to provide N2 to the plant when it would otherwise be unavailable.
- Nodules are growths on a plant’s roots that are caused by rhizobia and related bacteria.
- Both parties can benefit from this relationship: the plant can give nutrients and shelter for the bacterium, and the bacteria can provide the plant with nitrogen.
- N2 fixers from other organisms often partner up with plants.
- Grass roots, including their inner cells, the intercellular spaces between the cortex and endodermis, and the xylem cells themselves are all colonised by these bacteria.
- They may also supply chemicals that promote plant growth, in addition to the plant-available nitrogen.
2. Mycorrhizal Associations
- Mycorrhizal fungi develop a symbiotic relationship with plant roots without causing root disease.
- These fungi are found in the rhizospheres of the majority of plants and develop relationships with all gymnosperms, more than 83 percent of dicotyledonous plants, and 79 percent of monocotyledonous plants.
- Mycorrhizal fungus can form structures on the exterior (ectomychorrhizae) or the interior (endomycorrhizae) of plant roots.
- The hyphae of fungi permit the roots to contact a larger volume of soil.
- Some mycorrhizal fungi enhance the solubilization of nutrients like phosphorus.
- They aid the plant in enhancing nutrient absorption, particularly in stressed conditions (such as phosphorus- and water-deficient soils), selective ion uptake, and environmental protection.
- After colonisation by these fungi, plant exudation patterns may be altered, hence changing the microbial and macrofaunal communities of the rhizosphere.
- The fungi may also shield plant roots from disease invasion.
- Endomycorrhizal extraradicle hyphae release glomalin, a glycoprotein that aggregates soil particles, hence enhancing water-stable aggregates and soil structure.
- This relationship can boost the survival and growth of a plant, particularly in harsh or low-nutrient situations, and may have reforestation potential in damaged areas.
3. Plant Growth-Promoting Organisms
- Plant growth-promoting organisms are creatures that boost the development of plants in some way.
- Plant growth-promoting organisms are often particular bacteria or fungi that promote seed germination and plant growth.
- Many distinct systems are responsible for plant growth promotion.
- These organisms have been utilised in biological control programmes to defend plants against plant infections, as biofertilizers to fix atmospheric N2, and in phytostimulation, which directly enhances plant growth through the synthesis of plant growth regulators.
- Some may make nitrogen more available to plants and have the potential to minimise application of inorganic fertilisers.
- These organisms may create plant growth-stimulating chemicals such as gibberellic and indoleacetic acid.
- The reduction in pathogen burden may also be a mechanism of plant growth promotion.
- Iron-chelating chemicals termed siderophores can operate to make iron more available to an organism and less available to the plant pathogen, therefore harming the pathogen. Since iron is a critical nutrient in metabolism and a cofactor in enzyme activity, microbial growth may be regulated by these siderophores.
- The microbial communities that invade the inside of the root and create intimate relationships with the root are considered endophytes.
- Endophytic bacteria may boost plant growth, give disease resistance, and aid the plant in withstanding stressors such as drought.
- The success of endophytes in colonising the rhizosphere may be the ability of the microbe to compete with other bacteria on the exterior or internal sections of plant roots.
- Introduction of plant growth-promoting organisms may modify the overall composition of the microbial community, notably in the rhizosphere.
4. Plant Growth-Inhibiting Organisms
- It is possible for the rhizosphere to get colonised by growth-inhibiting organisms, which can then change plant development.
- Disease signs are not always present when plant growth is stunted.
- Many harmful organisms, like as fungus and bacteria, are found in the rhizosphere and prey on germinating seeds and young roots.
- It is possible that pathogens secrete extracellular enzymes that emulsify cell walls, releasing their contents for destruction.
- Toxins made by some infections alter membrane permeability or enzyme activity, both of which can disrupt a metabolic pathway. These microbe-produced poisons could be host-specific or have a broad range of plant family targets.
- Some pathogens create polysaccharides that can obstruct vascular tissues, leading to death.
- Having a tumor-inducing bacterial plasmid integrated into the plant genome causes gall formation in the roots by causing an overabundance of the hormones auxins and cytokinins.
- Root cell destruction and toxicity from nematode waste reduces the plant’s ability to take up water and nutrients.
- Other microbial infections may enter the roots through the puncture holes caused by eating.
- Plant growth can be stunted by deleterious rhizobacteria (DRB) even when there are no outward signs of disease.
- These organisms have been found on the root surface and maybe in the cortical intercellular spaces.
- The only food source for DRB is the organic substances generated by plant root cells; they do not parasitize the plant. Phytotoxins, plant growth regulators, volatile compounds, and antibiotics are only some of the metabolites they produce that have negative impacts on plant growth.
- They are generally found to be species or cultivar specific in their colonisation and inhibition, and root exudates may play a role in some plant-DRB interactions.
5. Bioremediation
- Over the course of the last few decades, pollution has increased as industrialization and agricultural intensification have both contributed to the release of toxic wastes into the biota of the planet.
- Due to its poisonous and carcinogenic nature, and tendency to bioaccumulate in people and other species, the prompt and cost-effective clean-up of polluted regions has become a concern.
- Bioremediation, which makes use of plants’ and microbes’ natural function in the transformation, mineralization, and complexation of organic and inorganic contaminants, can be improved by plant-microbial systems.
- When compared to more traditional methods of soil remediation, bioremediation is more cost-effective, makes use of less complicated equipment, and has a smaller environmental impact on the contaminated area.
- Bioremediation may be promoted by plant root exudates that demonstrate strong binding affinities to particular pollutants or by rhizosphere bacteria that mineralize toxins into their harmless derivatives.
- The microbial populations associated with plant roots may aid in the mineralization of potentially harmful substances in the soil.
- Several mechanisms have been proposed to account for the “rhizosphere impact” on bioremediation.
- To begin, it has been hypothesised that elevated populations and activities of xenobiotic degraders are prompted by carbon substrates from root systems.
- A second factor in the success of soil remediation efforts to eliminate heavy metals like mercury, selenium, and zinc is the presence of abundant populations of microbes.
- The role of bacteria and bacterial consortiums in bioremediation has received the most attention, but other organisms, such as fungus, actinomycetes, mycorrhizal associations, and even rhizosphere animals, have also demonstrated degradation capabilities.
- Elucidating the mechanisms involved in the repair of contaminated sites by plants and their related creatures is, without a doubt, the greatest challenge in bioremediation technology.
- This knowledge will enable for the selection and engineering of the optimal plant–microbe pairings and optimization of the remediation process.
6. Biological Control
- The rhizosphere is the site of biological control interactions that employ diseases, parasites, or other predators to decrease the population or activity of another organism.
- The three major biological control tactics are classical, inundative, and integrated management.
- The traditional method involves the release, diffusion, and self-reproduction of natural enemies against pests.
- In the rhizosphere, the inundative strategy involves the addition of a virulent strain of a biocontrol agent to inhibit pests. In this instance, the biocontrol agent is not self-sustaining and must be applied annually to the target host.
- The integrated management method is a comprehensive strategy that incorporates management actions to conserve or improve pests’ natural enemies.
- Pathogens, nematodes, and weeds can all be managed with biological control.
- Biological illness management provides alternatives to chemical disease management.
- The success of biocontrol techniques is aided by the interactions of organisms in the rhizosphere and the ecological significance of these interactions in the root and seed environment.
- The suppression of plant pathogens such as Fusarium oxysporum, Gaeumannomyces graminis, Pythium, and Phytophthora species by some soils is believed to be the result of physiochemical and microbiological soil variables.
- The suppression is targeted and may involve multiple methods. Antibiosis, formation of siderophores or volatile chemicals, parasitism, competition for nutrition, competition for ecological niches, and induced disease resistance are some of the mechanisms.
- Due to the formation of disease-suppressive soils, crop monoculture can, over time, result in a decline in disease.
- Crop rotation, soil amendments, cover crops, fumigation, and soil solarization are other management techniques that may affect the rhizosphere habitat and be considered as biocontrol methods.
- Pathogens of protozoa and nematodes that are fungal in nature can be employed for biological control of these plant pests.
- Mycelial development into cortical cells is believed to aid in the control of protozoa-caused clubroot disease.
- As they feed, plant-parasitic nematodes modify the exudate patterns of the host plant. This alters the microbial ecology of the rhizosphere at certain places, which influences the colonisation of fungal and bacterial nematode antagonists.
- Using nematode-trapping fungi to lower nematode populations is one of the most intriguing and extensively studied fungal interactions for biological control.
- These predatory fungi develop a variety of trapping structures, including constricting rings, sticky knobs, and hyphal meshes.
- However, the elements that produce major predatory behaviour must be investigated in greater depth.
What is Root Exudation?
- Due to a phenomenon known as Root exudation, intense microbial activity occurs.
- The organic and inorganic substances that diffuse out of the root during unfavourable conditions are known as Root exudates.
- Root exudates thereby create a network between plants and microbes. Root exudates are composed mostly of root secretions and root diffusates.
Classification of Root Exudates
Based on chemical composition, there are three categories of root exudates:
1. Organic compounds
- Carbon-based compounds are those in which carbon serves as the backbone molecule and is attached to other elements by covalent bonds (hydrogen, nitrogen, etc.).
- Example: Proteins, organic acids, vitamins, amino acids, sugars, flavonoids, nucleotides, enzymes, etc. are all examples of biomolecules.
2. Inorganic compounds
- To be more specific, they are compounds that contain either carbon or hydrogen, but not both.
- Example: Several substances, including water, anions, and the gases carbon, oxygen, nitrogen, etc., serve as examples.
3. Miscellaneous compounds
- Other than organic and inorganic compounds, some other substances are also released by the plant, which can impose a negative effect and called as miscellaneous compounds.
- Example: Auxins, glycosides, saponins, hydrocyanic acids, etc. are all examples of such chemicals.
Role of Root Exudates
- It’s a food source for the microorganisms.
- Plants’ roots produce exudates that prevent them from drying out.
- It helps keep the soil moist, which is essential for the development of soil microbes.
- It also safeguards the root from biotic stress, or pathogens, by the release of defence proteins and antimicrobial compounds.
- Systemic defences are bolstered in response to abiotic stresses including those posed by heat, salt, and acidity.
- It aids in soil aggregation by the release of mucilage.
- Soil chemistry and physics may be affected by:
- Osmotic pressure
- Ionic balance
- Redox potential
Factors Affecting Root Exudation
- Temperature and luminosity
- When a plant wilts, it produces an abundance of amino acids.
- The production of secondary metabolites by particular bacteria.
- Rhizospheric microorganisms capable of influencing root permeability and metabolism
Functions of rhizosphere microbiome
Positive effects
1. Effects of rhizosphere microorganisms on nutrient acquisition by plants
- The rhizosphere microbiome has a substantial impact on the nutritional status of plants.
- Rhizobia that fix nitrogen and mycorrhizal fungi that enhance phosphorus uptake are well-known examples.
- Microorganisms in the rhizosphere can also enhance the uptake of trace metals like as iron. Iron is abundant in soil, but under neutral to alkaline conditions, it largely occurs in the insoluble ferric oxide form, which is inaccessible to microbial development.
2. Supporting plant growth under biotic stress
- The rhizosphere is the first line of defence against soilborne infections for plant roots.
- Diverse members of the rhizosphere microbiome can combat soil-borne pathogens prior to and during initial infection, as well as during secondary dissemination on and within root tissue.
- Antibiosis, competition for trace elements, nutrients, and microsites, parasitism, interference with quorum sensing that affects virulence, and induced systemic resistance are the primary methods by which rhizosphere bacteria repel plant diseases.
- If not all, the vast majority of rhizobacteria produce compounds that limit the growth or activity of rival microbes.
- Rhizosphere microbiome members can also affect the plant immune system. In many instances, the systemic resistance response elicited in plants by beneficial rhizobacteria is controlled by the phytohormone jasmonic acid.
3. Supporting plant growth under abiotic stress
- It has been hypothesised that the rhizosphere microbiome contributes to the ability of certain plant species to survive in harsh environments.
- For instance, the soil isolate Achromobacter piechaudii ARV8, isolated from an arid and saline environment, dramatically boosted the biomass of tomato and pepper seedlings subjected to transitory drought stress.
- Rhizobacteria have been demonstrated to promote plant development under conditions of flooding.
- Low-temperature environments harbour microorganisms evolved to survive in such conditions. Despite the effect of low temperatures on nodule formation and nitrogen fixation, it is fascinating to note that native legumes in the high arctic may nodulate and fix nitrogen at comparable rates to those reported for legumes in temperate climes.
- pH and excessive concentrations of hazardous chemicals are two additional abiotic variables that may hinder plant growth. In numerous agricultural systems around the globe, low pH or contaminated soils pose significant obstacles. In the instance of pH stress, it was revealed that 2,4-diacetylphloroglucinol (DAPG)-producing Pseudomonas fluorescens strains dramatically decreased foliar lesions generated on corn growing in a low-pH environment.
Negative Effects
1. Fungi and oomycetes
- The germination, development, and establishment of fungal and oomycete pathogens in the rhizosphere depend on a variety of cues from the host plant.
- Several factors, including as changes in abiotic conditions (e.g. pH) and root exudates, can activate the dormancy of fungal spores.
- Low quantities of phenolic substances such as p-hydroxybenzoic, gallic, coumaric, cinnamic, ferulic, salicylic, and sinamic acids in root exudates enhanced conidial germination of pathogenic fungus, whereas higher concentrations had an inhibitory impact.
- Four phenolic acids from cotton root exudates inhibit Verticillium dahlia spore germination.
- Also, alkaloids derived from the roots of Veratrum taliense (Liliaceae) prevent the growth of Phytophthora capsici and Rhizoctonia cerealis.
- The composition of alkaloids in the roots and shoots of Jacobaea vulgaris was significantly influenced by soil type and soil microorganisms, particularly retrorsine and retrorsine N-oxide.
- Both alkaloids suppress the growth of the mycelium of various plant-associated fungi, including Fusarium oxysporum, Fusarium sambucinum, and Trichoderma sp. The influence of microbes on the alkaloid composition of plants may have additional ecological repercussions, as these modifications may attract specialised herbivores while discouraging generalists.
2. Nematodes
- The majority of nematodes in soil are free-living, but some feed on the root exterior (migratory ectoparasitic), others penetrate and travel within the root interior (migratory endoparasitic), and others establish a feeding site in the root where they reproduce (sedentary endoparasites).
- It is essential for plant-parasitic nematodes other than cyst or polyphagous root knot worms to use chemical gradients to locate their host plants.
- Their sensory equipment allows them to navigate, discover food sources, and orient themselves. In the physicochemically complex soil matrix, volatile and water-soluble chemicals are key nematode feeding cues.
- It has been postulated that volatile molecules play a significant role in long-range chemotaxis, but water-soluble compounds are more ideal for short-range chemotaxis.
3. Opportunistic human pathogens in the rhizosphere
- In addition to ‘real’ human infections such as Salmonella enterica serovar typhimurium and Escherichia coli O157:H7, the plant environment provides a haven for pathogens that exclusively infect immunocompromised or immunocompromised people.
- Several wild and cultivated plant species have been documented to harbour opportunistic human infections in the rhizosphere, including Burkholderia (ceno)cepacia, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia.
- In rhizosphere conditions, however, additional bacterial species that cause skin, wound, and urinary tract infections (such as Bacillus cereus and Proteus vulgaris) can also be detected.
4. Plant colonization by human pathogens
- Following attachment, human pathogenic bacteria, specifically Enterobacteriaceae, are able to infiltrate root tissue. For a more exhaustive list of instances of plant internalisation of human diseases.
- Unlike their invasion of animal hosts, enteric bacteria appear to primarily inhabit the apoplastic regions of plant hosts. At areas of lateral root emergence, human pathogenic bacteria reach the root tissue, according to numerous studies.
5. Soil health status and occurrence of potential human pathogens
- The rhizosphere of sugar beet seedlings contains various potentially harmful bacteria, including Achromobacter xylosoxidans, Alcaligenes faecalis, A. xylosoxidans, Janthinobacterium lividum, Enterobacter amnigenus, Serratia marcescens, Bacillus cereus, and Staphylococcus aureus.
- In contrast, Stenotrophomonas maltophilia was much more prevalent in suppressive rhizosphere soil than in supportive soil.
Lot going on out there we don't even realize. God set it up for us and gave us a chance to at least begin to understand. Amazing, life is also taking place underground. Plants communicate with one another and microbes through root exudates.
Agreed the synergy involved in god’s great creation is beyond our comprehension, but it's fun to tryLot going on out there we don't even realize
Those nugs look gorgeous Moose!!Princess Buttercup tester Twenty20 Mendocino. 42.2N 13 weeks of flower (dirty blonde x white truffle)
View attachment 19086950 View attachment 19086952 View attachment 19086953 View attachment 19086959
Should work no problem and you can't beat the sunsee what turns out!

Honestly, I haven't noticed much difference at all as long as most of the resin heads are cloudy That's with all my plants over the years , l almost always leave a few bud out to seeAwesome, I bet that resin is loaded with terpenes this time of the year!
Does potency peak earlier and then terpenes become more dominant with a more well rounded effect if harvested later?
Rain is setting up this week!
View attachment 19093621