so happy to hear from you Joe!  thought you'd gone missing as soon as i started experiments. Just wanted to thank you especially again for all the help you've given me, it meant i was able to get stuck straight into it when i had all the components to quickly figure it all out, and on this cancer rollercoaster my friend and i don't have time on our side so I can't elaborate enough how much I appreciate your generous and extremely valuable and insightful help, and your patience for putting up with all my newb questions!
thought you'd gone missing as soon as i started experiments. Just wanted to thank you especially again for all the help you've given me, it meant i was able to get stuck straight into it when i had all the components to quickly figure it all out, and on this cancer rollercoaster my friend and i don't have time on our side so I can't elaborate enough how much I appreciate your generous and extremely valuable and insightful help, and your patience for putting up with all my newb questions!
 Pretty much all my scans so far are either 2uL or 3uL, with just one or two 4uL's (ie. when only 3 lanes on full plate). In the case of that very latest run where i did the 3hr decarb each lane = 3uL, each 1.5ml eppendorf had exactly 50mg bud sample (half of what the commercial kits recommend), and exactly 1.0mL hexane so each tube looks about 2/3 full.
Pretty much all my scans so far are either 2uL or 3uL, with just one or two 4uL's (ie. when only 3 lanes on full plate). In the case of that very latest run where i did the 3hr decarb each lane = 3uL, each 1.5ml eppendorf had exactly 50mg bud sample (half of what the commercial kits recommend), and exactly 1.0mL hexane so each tube looks about 2/3 full.
 and it was cool to see how even other solvents like acetone and isopropyl alcohol were relatively useless by comparison, gave me more appreciation of having the right solvent for the job.
and it was cool to see how even other solvents like acetone and isopropyl alcohol were relatively useless by comparison, gave me more appreciation of having the right solvent for the job.
ps. Yes ive decided one more final night of experiments tonight!
#1) high THCV strain. Ive done recent tests with a high-CBG strain but want to try a high-THCV one (lanes 5 and 1 respectively).
#2) test if leaving the mobile phase to go into 'overtime' (for 5-10 mins after the solvent front has already reached the top) will result in better use of the limited plate space and provide better separation - or will it just make a mess of it all once the plate is fully waterlogged!? cant wait to find out!
#3) i want to try Fast Blue just in pure distilled water, no NaOH. Will it dissolve better!? will the plate still develop as well and vibrant and 'fix' the dye as well? another user said he started with NaOH but now doesnt bother, so thats a good sign at least that it should be ok!
All that and more in tonights episode of How Freaking Awesome Is Science(!)
next time i might try doubling the NaOH 1.0M solution from 4gms/L to 8gms/L, then only use half as much, and try dissolving Fast Blue in the other half (pure distilled water) before mixing ... worth a try anyway. Interestingly enough (or you might be thinking "obviously" lol) the Fast Blue did not want to dissolve at all in hexane! It certainly would be both easier and more efficient to get a full dissolve from the BB instead of straining with coffee filter so hopefully that'll work, but if not at least this backup of using coffee filter is still getting me nice reasonably clean results, phew!Zinc salts generally probably dissolve better in water than NaOH. The B salt dissolves in water easily.
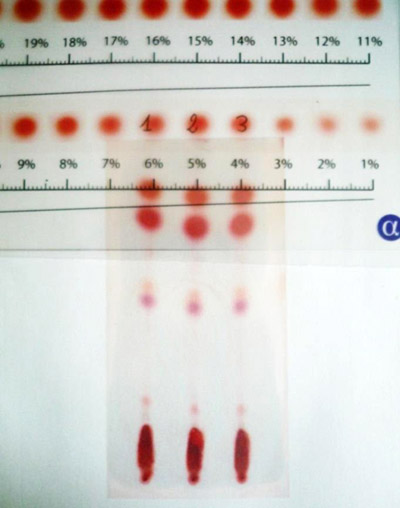
yes im so happy with the colors!! "Not only will we let you see all your cannabinoids, we'll make it stunningly BEAUTIFUL for you too!" - Mother Nature last Friday, lol. I know Chimera prefers UV, but gimme pretty colorful dots any day! I did read that BB is more vibrant than B though so maybe thats why, but other than that single brief mention i've never seen any written comparisons between the two sadly. Would you say vibrancy is the only main functional difference? The scanner does seem to give a slight color boost but very mild. And remember the very first experiment I did was to determine how much (how little as it turns out) to use, so I'm pretty sure I'm not using too much, and if you can tell by how long i had to wait for free shipping i'm a cheapskate and FBBB isn't cheap so don't want to use any more than needed, lolYou are getting a lot of color for 3 uL. 5 is much paler for me with B but the extract is dilute. Have you tried a more dilute load?
I'm so happy at the separation we're still able to get from our non-fancy mixes though, maybe won't hold up for HP but easily good enough for home hacks like me, phew!Having high-performance reversed-phase plates would make for good separations, yes. This is about the same as HPLC, which normally uses such fancy materials.
so the $64,000 question (might be a bit more in 2016) - how long and at what temp do you prefer to decarb buds?500. It was never presented by the person that posted it as the time and temperature that you need to have buds in your oven. The graphs show certain general trends and knowing what's going on is what's important. GW Pharma has their advice on large quantities and the different cannabinoids and that's all posted here a long time ago. The different acids have different stabilities and Mechoulam says THCA-B melts at 184.
ps. Yes ive decided one more final night of experiments tonight!
#1) high THCV strain. Ive done recent tests with a high-CBG strain but want to try a high-THCV one (lanes 5 and 1 respectively).
#2) test if leaving the mobile phase to go into 'overtime' (for 5-10 mins after the solvent front has already reached the top) will result in better use of the limited plate space and provide better separation - or will it just make a mess of it all once the plate is fully waterlogged!? cant wait to find out!
#3) i want to try Fast Blue just in pure distilled water, no NaOH. Will it dissolve better!? will the plate still develop as well and vibrant and 'fix' the dye as well? another user said he started with NaOH but now doesnt bother, so thats a good sign at least that it should be ok!
All that and more in tonights episode of How Freaking Awesome Is Science(!)
Last edited: