Home TLC Thin layer chromatography
- Thread starter Thread starter NotGuilty
- Start date Start date
EXPERIMENT NIGHT #3: DECARBOXYLATION
so when trying to figure out how long to decarb for, it seems 120C/248F for 1hr is ideal for both THC and CBD? can only go from what ive read, lol. Anyway my experiment tonight seems to back that up.
I took some bud from some random recreational strain (so expecting high THC low/no CBD) and made 9 x 70mg portions of it, and wrapped each one individually in a square of alfoil about the size of a cig lighter:

Then put all of them in my oven, one of these air forced convection style ones - yes the alfoil pieces were slowly blowing around the the whole time, only at the base though so no direct contact issue. I had it set to 125C (error range lets say 120-130), which was its lowest marked setting.

Then after 5 minutes i took the first out, then after 10 mins took the next out, then after 20 took the next out, etc etc every 10 mins until the last one came out after 80 minutes.

so when trying to figure out how long to decarb for, it seems 120C/248F for 1hr is ideal for both THC and CBD? can only go from what ive read, lol. Anyway my experiment tonight seems to back that up.
I took some bud from some random recreational strain (so expecting high THC low/no CBD) and made 9 x 70mg portions of it, and wrapped each one individually in a square of alfoil about the size of a cig lighter:

Then put all of them in my oven, one of these air forced convection style ones - yes the alfoil pieces were slowly blowing around the the whole time, only at the base though so no direct contact issue. I had it set to 125C (error range lets say 120-130), which was its lowest marked setting.

Then after 5 minutes i took the first out, then after 10 mins took the next out, then after 20 took the next out, etc etc every 10 mins until the last one came out after 80 minutes.

miltiano32
Member
I usually decarb for 30min at about 240degrees. Looking at your image it would seem that the 30min interval was higher? or am i interpreting it wrong?
Look at the THCA levels though when you compare 30 mins to 60 mins, still lotsss of THCA left to convert to THC at 30 mins, but seems to hit the sweet spot at 60 mins.
So it seems pretty clear with THCA at least. But i agree the THC spots themselves are trickier to interpret!
I cut them out (being careful to try to avoid the crossover from THC to THCV spots), and brought them into JustTLC for a closer look (btw keep in mind each sample is 3 x 1uL microliter so its very tricky to get them all exact, so some individuals may stick out, but you can still clearly see the trend over multiples)

Note that the ID Numbers given by JustTLC are random but the lane positions are still the same -- the 0 minute (cold) run on the left as #10, then the 5-minute run next to that as #8, etc etc all the way to #4 which is the 80-minute run.
So it seems pretty clear with THCA at least. But i agree the THC spots themselves are trickier to interpret!
I cut them out (being careful to try to avoid the crossover from THC to THCV spots), and brought them into JustTLC for a closer look (btw keep in mind each sample is 3 x 1uL microliter so its very tricky to get them all exact, so some individuals may stick out, but you can still clearly see the trend over multiples)

Note that the ID Numbers given by JustTLC are random but the lane positions are still the same -- the 0 minute (cold) run on the left as #10, then the 5-minute run next to that as #8, etc etc all the way to #4 which is the 80-minute run.
Last edited:
only 25mg? i see you didn't shop around! I'd cancel that order if it's not too late. That's about all you get in those commercial test kits and they're about the same price! That amount is only good for about two baths or ~3-10 plates. Should be able to get at least 250mg for $100 - I got 5000mgs for just a few hundred and that was imported from a Japanese lab and freighted with dry ice. Take some time and shop around with googlealso email some local labs and ask them how much to get some for you (worked for me). Dont forget Fast Blue B is the other option to BB, its usually a bit cheaper too and only requires refrigeration not freezer, but its more of a carcinogen concern and results not as vibrant (please read this thread in full if you havent yet)
I'm impressed with the definition you're getting with the Fast Blue BB, thanks again for sharing your discoveries!
So, I looked for Fast Blue BB here in the US, is this about where you'd need go if you don't have credentials?
http://www.labdepotinc.com/p-17299-fast-blue-bb-salt.php
ps. there is every chance i accidentally mixed the 5 and 10 minute runs up - not just because the TLC result suggests thats the case, but because i had just the two of them laid out on my bed when a car with siren drove past so i kneeled on the bed to look out the window, which caused the two alfoil portions to come together lol. So I would say that, looking at the TLC result, it's probably safe to assume that that is indeed the case - it's the only real anomaly afterall. So, just switch the 5 and 10 minute lanes around.
 and its so simple yet so powerful - the results reveal all! Grow out 10 plants and wonder why that 1 is a keeper? you'll soon know!!! lol, omg HOW GOOD IS SCIENCE KIDS!?!?
and its so simple yet so powerful - the results reveal all! Grow out 10 plants and wonder why that 1 is a keeper? you'll soon know!!! lol, omg HOW GOOD IS SCIENCE KIDS!?!?
btw correct solvent also required to get good definition! im using 4:1 hexane:diethyl ether with great success, which is the one (out of 6 tested) that was suggested as giving best cannabinoid separation.
But tonight in Experiment Night #4 or 5 or whatever im up to haha I'm going to try lots of different eluents, including chloroform which is also supposed to give excellent definition but I also plan to try a few others, including the probably-not-so-universal solvent: distilled water. Not expecting great results from that, but expecting great science
but I also plan to try a few others, including the probably-not-so-universal solvent: distilled water. Not expecting great results from that, but expecting great science 

Anyway, TONIGHTS ROUND OF EXPERIMENTS STARTS NOW!!
yes it seems true that BB gives a more "vibrant" result than B, I'm not sure if that makes it any more useful though, but hey its a thing of visual beauty to meI'm impressed with the definition you're getting with the Fast Blue BB, thanks again for sharing your discoveries!
btw correct solvent also required to get good definition! im using 4:1 hexane:diethyl ether with great success, which is the one (out of 6 tested) that was suggested as giving best cannabinoid separation.
But tonight in Experiment Night #4 or 5 or whatever im up to haha I'm going to try lots of different eluents, including chloroform which is also supposed to give excellent definition
I don't know about US but I don't think you need any credentials (or whatever) at all to get Fast Blue B or BB, it's essentially just a dye that you can use to test phenolic compounds such as those in strawberries (just google: "Fast Blue" phenolics -cannabinoids) so it's not cannabis-specific, and it's not on any "lists"So, I looked for Fast Blue BB here in the US, is this about where you'd need go if you don't have credentials?
Anyway, TONIGHTS ROUND OF EXPERIMENTS STARTS NOW!!
They're so famous for not selling things. It would be a big surprise if they would send you anything. A VWR is probably about the biggest size of supplier that might sell chemicals to individuals and/or deliver to residences.
Google gave me a few sources for the BB last week - additional search terms might bring up more. It will not arrive cold and may have been made in India and never refrigerated. Google just might give you a source for the B salt on the first hit.
Oh and what I said about ether is after learning everything you need to know about dealing with peroxide formation and the ease of ignition. Once you've got that down it's no big deal. Except for the penetrating smell freaking out neighbors.
I don't know about US but I don't think you need any credentials (or whatever) at all to get Fast Blue B or BB, it's essentially just a dye that you can use to test phenolic compounds such as those in strawberries (just google: "Fast Blue" phenolics -cannabinoids) so it's not cannabis-specific, and it's not on any "lists"
Anyway, TONIGHTS ROUND OF EXPERIMENTS STARTS NOW!!
Couldn't even find Fast Blue B, let alone Fast Blue BB at eBay...
Sigmund-Aldrich, India, China, and the previous linked source were about all I was coming up with for Fast Blue BB with a Google Search, just as G.O. Joe suggested in the quote above. The linked source seems like a zero questions asked supplier, but it would be nice to know there are other US sources for price comparison, and with maybe a smaller quantity option, like the five grams you purchased.
you wont find it at ebay although i did see it once a month ago, and Sigma only sell to registered unis/govt/LEO etc so they're a no-go (pretty sure u cant even buy distilled water from them without being reg'd), but Fast Blue itself isn't a restricted product so all you have to do is ask one of your local labs to import it for you, that worked for me. There are plenty of labs that sell both B and BB, mainly in China though i got mine from Japan. And remember just because you can't find an "Add To Cart" option doesn't mean its not available via an email either  keep shopping around, take the time and dont give up, you'll find some and at good price - "the chase" is worth it! I dont know how much B is used (seems similar) but 20mgs of BB seems about right for 1 bath, which is enough for at least three full plates, so should get approx 50 baths out of 1000mgs. Do try and get BB if you can if only because its considered safer than B in regards to carcinogen, and you have to deal with it as a powder every time at least initially before you solute the bath.
keep shopping around, take the time and dont give up, you'll find some and at good price - "the chase" is worth it! I dont know how much B is used (seems similar) but 20mgs of BB seems about right for 1 bath, which is enough for at least three full plates, so should get approx 50 baths out of 1000mgs. Do try and get BB if you can if only because its considered safer than B in regards to carcinogen, and you have to deal with it as a powder every time at least initially before you solute the bath.
Last edited:
Final night of experiments: ELUENTS!
Im sad to say this is my final night of experiments, as I've figured it out enough to be able to hand it all over to my friend this weekend so she can start using it straight away without wasting any time, as she starts her quest to find CBD-rich strains to help with her battle against cancer. Just another quick THANKYOU to everyone who has helped me along the way, both through privmsg and through this thread! If you're reading this now and find it interesting but haven't yet read this thread from page 1, please stop now and do so! it took me six weeks to figure all this shit out haha, but itll take you 10 minutes to read this thread
THE ELUENT!
By eluent i am refering to the mobile phase - the solution used for the capillary action. For the extract im still just using pure hexane, which from what ive read seems to be the most common used for extraction.
Anyway if you're one of the three people following my progress in this thread lol you would've noticed i'm using 4:1 hexane:diethyl ether as my eluent because 1) i'd seen hexane used in a lot of the papers, 2) both hexane and diethyl ether didnt appear to be on any 'lists', but mainly because 3) one of the papers compared 6 different eluents (see earlier post for details - chloroform wasn't one of them btw), and found that the hexane diethyl mix achieved the best separation of cannabinoids.
So it seems there are various solvents that can be used, but only a few that achieve that desired separation really well.
I was curious to see how some other solvents would perform as the eluent!
So i cut an aluminium TLC plate up into 4 pieces... in future tho i think 3 is better, as theyre so thin with 4 cuts that its tricky to stand then up straight in the round jar, especially when the foil bends a bit as it easily does then. Because theyre too thin 1) some edge effect happened, and 2) not all wanted to follow the plate because it wasnt completely vertical.
So these are pretty rough! but still give a good enough approximation of how useless these solvents are - you can see the hexane:diethyl is the only one offering cannabinoid separation - with the other three its difficult to tell whether the sample has THC, CBD or both:

I was inspired by this where they used chloroform for the eluent and also got good results.
So in the name of science i bought a small bottle of chloroform!
Two advantages chloroform has over hexane:diethyl ether ...
1) its a single solution, no need to spend any time measuring out a mix.
2) it doesnt have that "get the fuck out of here before i assault your nose" kick in the face that diethyl ether has ... WOW that stuff has some hardcore fumes and makes it hard to work with. Hexane and chloroform on the other hand are both much milder in that respect.
But how does it perform? I did a side-by-side comparison with hexane:diethyl ether, using 5 different bud samples that i had decarb'd @ 125C for 60mins:

Each row on each plate is the same strain/sample (you can see for example it appears that lane 5 on both has the most CBG)
But it appears that the chloroform is giving the greatest cannabinoid separation!? ... and actually seems to be getting greater use out of the expensive 10cm x 5cm real estate (yes $2 a plate is expensive)
(yes $2 a plate is expensive)
Joe i'd love to hear your thoughts about this
Im sad to say this is my final night of experiments, as I've figured it out enough to be able to hand it all over to my friend this weekend so she can start using it straight away without wasting any time, as she starts her quest to find CBD-rich strains to help with her battle against cancer. Just another quick THANKYOU to everyone who has helped me along the way, both through privmsg and through this thread! If you're reading this now and find it interesting but haven't yet read this thread from page 1, please stop now and do so! it took me six weeks to figure all this shit out haha, but itll take you 10 minutes to read this thread
THE ELUENT!
By eluent i am refering to the mobile phase - the solution used for the capillary action. For the extract im still just using pure hexane, which from what ive read seems to be the most common used for extraction.
Anyway if you're one of the three people following my progress in this thread lol you would've noticed i'm using 4:1 hexane:diethyl ether as my eluent because 1) i'd seen hexane used in a lot of the papers, 2) both hexane and diethyl ether didnt appear to be on any 'lists', but mainly because 3) one of the papers compared 6 different eluents (see earlier post for details - chloroform wasn't one of them btw), and found that the hexane diethyl mix achieved the best separation of cannabinoids.
So it seems there are various solvents that can be used, but only a few that achieve that desired separation really well.
I was curious to see how some other solvents would perform as the eluent!
So i cut an aluminium TLC plate up into 4 pieces... in future tho i think 3 is better, as theyre so thin with 4 cuts that its tricky to stand then up straight in the round jar, especially when the foil bends a bit as it easily does then. Because theyre too thin 1) some edge effect happened, and 2) not all wanted to follow the plate because it wasnt completely vertical.
So these are pretty rough! but still give a good enough approximation of how useless these solvents are - you can see the hexane:diethyl is the only one offering cannabinoid separation - with the other three its difficult to tell whether the sample has THC, CBD or both:

I was inspired by this where they used chloroform for the eluent and also got good results.
So in the name of science i bought a small bottle of chloroform!
Two advantages chloroform has over hexane:diethyl ether ...
1) its a single solution, no need to spend any time measuring out a mix.
2) it doesnt have that "get the fuck out of here before i assault your nose" kick in the face that diethyl ether has ... WOW that stuff has some hardcore fumes and makes it hard to work with. Hexane and chloroform on the other hand are both much milder in that respect.
But how does it perform? I did a side-by-side comparison with hexane:diethyl ether, using 5 different bud samples that i had decarb'd @ 125C for 60mins:

Each row on each plate is the same strain/sample (you can see for example it appears that lane 5 on both has the most CBG)
But it appears that the chloroform is giving the greatest cannabinoid separation!? ... and actually seems to be getting greater use out of the expensive 10cm x 5cm real estate
Joe i'd love to hear your thoughts about this
Last edited:
PDX Dopesmoker
Active member
EXPERIMENT NIGHT #3: DECARBOXYLATION
so when trying to figure out how long to decarb for, it seems 120C/248F for 1hr is ideal for both THC and CBD? can only go from what ive read, lol. Anyway my experiment tonight seems to back that up.
I took some bud from some random recreational strain (so expecting high THC low/no CBD) and made 9 x 70mg portions of it, and wrapped each one individually in a square of alfoil about the size of a cig lighter:
View Image
Then put all of them in my oven, one of these air forced convection style ones - yes the alfoil pieces were slowly blowing around the the whole time, only at the base though so no direct contact issue. I had it set to 125C (error range lets say 120-130), which was its lowest marked setting.
View Image
Then after 5 minutes i took the first out, then after 10 mins took the next out, then after 20 took the next out, etc etc every 10 mins until the last one came out after 80 minutes.
View Image
Oh wow thats fantastic, not only is it one of the coolest things I've seen on the internet in a long time, but it seems like it correlates pretty roughly to the skunkpharm decarbing chart too.

Do a CBD one! The general public doesn't currently have a good metric for how to decarb CBD (or if they do I haven't been paying attention)
glad to hear at least 1 other person finds this interesting hehe. I think the word "chromatography" scares off a lot of people, but this type of basic TLC really is something anyone can do from home. Unfortunately though I can't just "do a CBD one" sorry as I don't have any high-CBD plant material to work with (hopefully that will change now that my friend has this amazing tool). All i've got is some already-decarb'd CBD oils. But, quite a few texts I saw suggested that for both THC and CBD that 60mins @ 120C was ideal - the declining THCA level seems to suggest that too.
Im not sure how well it correlates to the Journal of Chromatography graph though (i had posted that previously in this thread) as i was doing flower samples, whereas they were doing 1) tiny dots of probably just 1-5 microliters, of 2) hexane extract, on 3) glass plates, and their graph seems to suggest ~30mins @ 122C is ideal, and interestingly i've come across quite a few websites that "debunk" (well, more explain than debunk) that graph for that reason - their test is different to what most people are doing at home.
Im not sure how well it correlates to the Journal of Chromatography graph though (i had posted that previously in this thread) as i was doing flower samples, whereas they were doing 1) tiny dots of probably just 1-5 microliters, of 2) hexane extract, on 3) glass plates, and their graph seems to suggest ~30mins @ 122C is ideal, and interestingly i've come across quite a few websites that "debunk" (well, more explain than debunk) that graph for that reason - their test is different to what most people are doing at home.
Last edited:
great thread



btw from what im reading it seems methanol might be a good way to get more out of the chloroform by dilution, as methanol is very cheap and it keeps popping up in my chloroform cannabinoid searches (for example one columnar chromatography paper used a 9:1 methanol:chloroform ratio!). Chloroform prices arent too bad, but nowhere near as cheap as methanol, so it would be interesting to see how much the chloroform can be diluted while still retaining good performance and cannabinoid separation. I'll have to personally give that experiment a miss for now but will certainly be recommending my friend try it, it could be a good cost saving measure, and if it doesn't work they've only wasted ~$10
[update] btw yes on page 6 of this thread Joe says "A nonflammable option is chloroform with just a little bit of methanol".
ps. ok one more final round of experiments tonight, lol -- I was really impressed with the chloroform, so I want to test how well CBD and THC are separated by chloroform, which we can't tell from that previous run as the samples were recreational strains with no detectable level of CBD. The samples tonight (made 2-3 nights ago) are a CBD paste which showed a beautiful spectrum (see earlier run), some RSO, a brownie, and a 'soup mix' of several samples to help show as many cannabinoids as possible. They looked great after hexane:diethyl, im very interested to see how they'll turn out after chloroform! AND im also doing another decarboxylation run! essentially the same as the previous run, but chloroform instead of hexane:diethyl ether. I'm also going to dunk a small whole bud into the Fast Blue bath! well, i wont be able to submerge it but i'll flatten it and do both sides. Can't wait to see how it all turns out ... will post results in a couple hours
[update] btw yes on page 6 of this thread Joe says "A nonflammable option is chloroform with just a little bit of methanol".
ps. ok one more final round of experiments tonight, lol -- I was really impressed with the chloroform, so I want to test how well CBD and THC are separated by chloroform, which we can't tell from that previous run as the samples were recreational strains with no detectable level of CBD. The samples tonight (made 2-3 nights ago) are a CBD paste which showed a beautiful spectrum (see earlier run), some RSO, a brownie, and a 'soup mix' of several samples to help show as many cannabinoids as possible. They looked great after hexane:diethyl, im very interested to see how they'll turn out after chloroform! AND im also doing another decarboxylation run! essentially the same as the previous run, but chloroform instead of hexane:diethyl ether. I'm also going to dunk a small whole bud into the Fast Blue bath! well, i wont be able to submerge it but i'll flatten it and do both sides. Can't wait to see how it all turns out ... will post results in a couple hours
Last edited:
Couldn't even find Fast Blue B, let alone Fast Blue BB at eBay...
Sigmund-Aldrich, India, China, and the previous linked source were about all I was coming up with for Fast Blue BB with a Google Search, just as G.O. Joe suggested in the quote above. The linked source seems like a zero questions asked supplier, but it would be nice to know there are other US sources for price comparison, and with maybe a smaller quantity option, like the five grams you purchased.
you wont find it at ebay although i did see it once a month ago, and Sigma only sell to registered unis/govt/LEO etc so they're a no-go (pretty sure u cant even buy distilled water from them without being reg'd), but Fast Blue itself isn't a restricted product so all you have to do is ask one of your local labs to import it for you, that worked for me. There are plenty of labs that sell both B and BB, mainly in China though i got mine from Japan. And remember just because you can't find an "Add To Cart" option doesn't mean its not available via an email eitherkeep shopping around, take the time and dont give up, you'll find some and at good price - "the chase" is worth it! I dont know how much B is used (seems similar) but 20mgs of BB seems about right for 1 bath, which is enough for at least three full plates, so should get approx 50 baths out of 1000mgs. Do try and get BB if you can if only because its considered safer than B in regards to carcinogen, and you have to deal with it as a powder every time at least initially before you solute the bath.
Would you please consider staying on this a little longer? I'd love to see your comparison of B versus BB, the price differential is huge...
$32/25g versus $450/25g
http://www.himediastore.com/search/?q=fast blue b
Also, I'd like to see more side by side raw versus decarbed results, as pure THC-A extract is the holy grail at the moment.
 Thanks again, you rocked it!!!
Thanks again, you rocked it!!!im not going anywhere, but my kit is going to my friend so tonight is my final run of experiments, for now at least. I personally dont want B because BB was made to replace it specifically because of carcinogen concerns - and im using TLC because my friend is chasing CBD because of cancer already. I wouldve got B if i couldnt find BB but it turned out easy enough to get BB so I wont be getting any B for side-by-side comparisons or whatever. B does seem easier/cheaper to get though + easier to store in fridge instead of freezer (and then less time waiting for vial to reach room temp to minimise moisture). BB is also a suspected carcinogen anyway lol, so i guess as long as you treat them both with respect and use appropriate safety gear then it may not matter which you use, and it does seem they're pretty much perfectly interchangeable. I personally highly encourage using a bath instead of aerial spray regardless which one you use!!
My final experiment results within the next hour... 1) decarboxylation but with chloroform, 2) another samples mix but this one with high CBD, and 3) a BUD dunked into the dye!!
Watch this space
My final experiment results within the next hour... 1) decarboxylation but with chloroform, 2) another samples mix but this one with high CBD, and 3) a BUD dunked into the dye!!
Watch this space
Final night of experiments: CHLOROFORM ELUENT TESTS!
But before the tests, ever wondered what would happen to a bud if we dunked it into the Fast Blue dye!? ok just me then! Anyway a freshly made bath of Fast Blue BB is fluro-yellow, similar to vitamin-rich urine, but over an hour or two quickly degrades to a browny color with a red tinge, and at that stage it's very weak - make another batch. Anyway as soon as i put the bud in the bath quickly turned almost blood-red, no surprise being a high THC strain, so the end result is quite predictable i guess ... (and a high-CBD bud should dye orange):

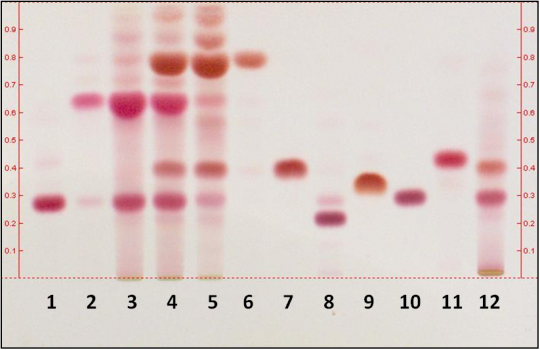
On with the experiments ... so I wanted to see how well chloroform would perform, in particular with separation of THC and CBD. You can see in this image where somebody also used hexane as the solvent and chloroform as the eluent and they got a nice separation of THC and CBD - you can see 6 of their 8 Fruity Jack samples have high CBD (their photo so credit to them):

Anyway I'll repost the ones I did for hexane:diethyl the other nights alongside the ones I just did tonight with chloroform.
Analysis of 1) high-THC RSO, 2) CBD paste, and 3) a mix of the two (so we should see both THC and CBD):

The DECARBOXYLATION runs are interesting, but I was surprised the chloroform one is tricky to decipher ...


NOTES:
1) for the Chloroform run, I had to change the Fast Blue bath over for the second plate, which is why the colors are a bit different (and you can see from the dots i didnt filter it as well either).
2) Spillage: a tiny bit of bud was stuck to the micropipette and dropped off onto the TLC plate while i was adding the drops, but as you can see fortunately it didnt end up interfering.
3) i used the same strain for both hexane & chloroform runs, but in the chloroform run i also added a second strain, which is the one from lane #5 in the mixed samples one from yesterday that showed high levels of CBG as i thought itd be interesting to see .... it seems like in the 0 minute (cold) lane you can see a lot of yellow at the base, then by 10 minutes thats nearly gone but there's a big orange CBG spot evolving, so maybe thats CBGA -> CBG in progress
4) despite being 10 degrees hotter @ 135C, and 10 mins longer @ 90 mins, i was surprised that this chloroform result isn't really showing signs of degradation
5) all were done in the same jar, but its strange how the chloroform runs are very sensitive to the shape of the jar (you can see the curve in the results on each side like \___/ instead of -----), whereas the hexane runs look more like they're done on a flat bottom jar. I am still on the hunt for a truly flat-bottom jar, preferably rectangle shaped!
CONCLUSION:
Both the chloroform and hexane:diethyl-ether mixes appear to have their own pros and cons, but both provide great separation. Ok cool - i'll just use them both to get an even better overall picture!
But before the tests, ever wondered what would happen to a bud if we dunked it into the Fast Blue dye!? ok just me then! Anyway a freshly made bath of Fast Blue BB is fluro-yellow, similar to vitamin-rich urine, but over an hour or two quickly degrades to a browny color with a red tinge, and at that stage it's very weak - make another batch. Anyway as soon as i put the bud in the bath quickly turned almost blood-red, no surprise being a high THC strain, so the end result is quite predictable i guess ... (and a high-CBD bud should dye orange):

On with the experiments ... so I wanted to see how well chloroform would perform, in particular with separation of THC and CBD. You can see in this image where somebody also used hexane as the solvent and chloroform as the eluent and they got a nice separation of THC and CBD - you can see 6 of their 8 Fruity Jack samples have high CBD (their photo so credit to them):

Anyway I'll repost the ones I did for hexane:diethyl the other nights alongside the ones I just did tonight with chloroform.
Analysis of 1) high-THC RSO, 2) CBD paste, and 3) a mix of the two (so we should see both THC and CBD):

The DECARBOXYLATION runs are interesting, but I was surprised the chloroform one is tricky to decipher ...


NOTES:
1) for the Chloroform run, I had to change the Fast Blue bath over for the second plate, which is why the colors are a bit different (and you can see from the dots i didnt filter it as well either).
2) Spillage: a tiny bit of bud was stuck to the micropipette and dropped off onto the TLC plate while i was adding the drops, but as you can see fortunately it didnt end up interfering.
3) i used the same strain for both hexane & chloroform runs, but in the chloroform run i also added a second strain, which is the one from lane #5 in the mixed samples one from yesterday that showed high levels of CBG as i thought itd be interesting to see .... it seems like in the 0 minute (cold) lane you can see a lot of yellow at the base, then by 10 minutes thats nearly gone but there's a big orange CBG spot evolving, so maybe thats CBGA -> CBG in progress
4) despite being 10 degrees hotter @ 135C, and 10 mins longer @ 90 mins, i was surprised that this chloroform result isn't really showing signs of degradation
5) all were done in the same jar, but its strange how the chloroform runs are very sensitive to the shape of the jar (you can see the curve in the results on each side like \___/ instead of -----), whereas the hexane runs look more like they're done on a flat bottom jar. I am still on the hunt for a truly flat-bottom jar, preferably rectangle shaped!
CONCLUSION:
Both the chloroform and hexane:diethyl-ether mixes appear to have their own pros and cons, but both provide great separation. Ok cool - i'll just use them both to get an even better overall picture!
Last edited:
another eluent to try (not me i've already spent enough on chloroform + hexane/diethyl, lol), and seemingly very affordable and relatively safe is: 0.1% glacial acetic acid in a mixture of methanol and water (75:25)
That's what Chimera said earlier in this thread that he uses, and that's what Uni Mississippi used to produce this TLC, again some really nice separation
src http://www.botanicalauthentication.org/index.php/Cannabis_spp._(pistillate_inflorescence_and_leaf)
That's what Chimera said earlier in this thread that he uses, and that's what Uni Mississippi used to produce this TLC, again some really nice separation

src http://www.botanicalauthentication.org/index.php/Cannabis_spp._(pistillate_inflorescence_and_leaf)
Another night of experiments: DECARBOXYLATION OVER 3HRS, VISUALISED WITH BOTH ELUENTS!
My friend wasn't available to meet up over the weekend so i've still got the TLC kit for at least another day or two, possibly the week, although i've pretty much done all the experiments i wanted.
But as the Chloroform was new i wanted to do another run with that, and i also wanted to do another decarboxylation run, but longer, so this time I did 15 minute intervals, and actually took the last one an hour so 3 hours all up.
Anyway here's the result! Again i used the high-CBG strain as I don't have any high-CBD strains so this is next best besides my otherwise boring "THC-only" rec strains.


I was surprised there wasn't much difference between the 2hrs and 3hrs samples! But it does seem to show (especially on the hexane one) that 1hr:15 - 1hr:45 minutes was best to maximise THC levels before THC levels started degrading.
It's also very interesting how the chloroform eluent is very sensitive to the shape of the jar (just regular jar - not perfectly flat bottom), but the hexane:diethyl eluent isnt! Both were in exactly the same jar, with exactly 10mL of eluent. That jar-shape-sensitivity sadly makes the result more difficult to read, but im assuming a flat-bottomed jar would easily solve the problem.
The solvent front was the very top of the plate, for all plates. (So yes - different eluents = different Rf values!). If i do another round of tests i'd like to let the hexane one run for another 5-10 minutes longer (after the solvent front has reached the top) just to see if it makes better use out of the limited real estate like the chloroform one does.
So anyway 1.5hrs @ 125ºC/257ºF is looking like a winner!
My friend wasn't available to meet up over the weekend so i've still got the TLC kit for at least another day or two, possibly the week, although i've pretty much done all the experiments i wanted.
But as the Chloroform was new i wanted to do another run with that, and i also wanted to do another decarboxylation run, but longer, so this time I did 15 minute intervals, and actually took the last one an hour so 3 hours all up.
Anyway here's the result! Again i used the high-CBG strain as I don't have any high-CBD strains so this is next best besides my otherwise boring "THC-only" rec strains.


I was surprised there wasn't much difference between the 2hrs and 3hrs samples! But it does seem to show (especially on the hexane one) that 1hr:15 - 1hr:45 minutes was best to maximise THC levels before THC levels started degrading.
It's also very interesting how the chloroform eluent is very sensitive to the shape of the jar (just regular jar - not perfectly flat bottom), but the hexane:diethyl eluent isnt! Both were in exactly the same jar, with exactly 10mL of eluent. That jar-shape-sensitivity sadly makes the result more difficult to read, but im assuming a flat-bottomed jar would easily solve the problem.
The solvent front was the very top of the plate, for all plates. (So yes - different eluents = different Rf values!). If i do another round of tests i'd like to let the hexane one run for another 5-10 minutes longer (after the solvent front has reached the top) just to see if it makes better use out of the limited real estate like the chloroform one does.
So anyway 1.5hrs @ 125ºC/257ºF is looking like a winner!
So it turns out just shaking it harder in a jar doesn't make some of the clumpy bits dissolve ... I wonder if it's because im trying to dissolve them in NaOH 0.1N solution - perhaps dissolving in water first is best?
Zinc salts generally probably dissolve better in water than NaOH. The B salt dissolves in water easily. You are getting a lot of color for 3 uL. 5 is much paler for me with B but the extract is dilute. Have you tried a more dilute load?
Joe i'd love to hear your thoughts about this
btw i wonder what dye might be used to show terpenes?
Probably not much is terpene specific but this and the acetic acid it's already come up Hazekamp 2005 again -
Samples in ethanol were spotted on 10 x 20 cm silica plates. Two different TLC systems were used. For the non-polar system, reversed phase (C18) silicagel plates F254 No. 105559 (Merck, Darmstadt, Germany) were used with methanol/5% acetic acid 19 : 1 (v/v) as the eluent. For the polar system, normal phase silicagel plates F254 No. 105554 (Merck, Darmstadt, Germany) were used with chloroform/methanol 19 : 1 (v/v) as the eluent.
Plates were developed in saturated normal chambers (saturation time 15 minutes). Absorption of chromatographic spots was evaluated under UV 254 nm. General visualisation of compounds was done by spraying with modified anisaldehyde-sulphuric acid spray reagent. For selective visualisation of cannabinoids, the TLC plate was sprayed with 0.5% fast blue B salt (o-dianisidine-bis-(diazotized)-zinc double salt) (Sigma) in water, followed by 0.1M NaOH.
Having high-performance reversed-phase plates would make for good separations, yes. This is about the same as HPLC, which normally uses such fancy materials.
i was doing flower samples, whereas they were doing 1) tiny dots of probably just 1-5 microliters, of 2) hexane extract
500. It was never presented by the person that posted it as the time and temperature that you need to have buds in your oven. The graphs show certain general trends and knowing what's going on is what's important. GW Pharma has their advice on large quantities and the different cannabinoids and that's all posted here a long time ago. The different acids have different stabilities and Mechoulam says THCA-B melts at 184.





