acespicoli
Well-known member
From a DJ Short article:
Your friend the freezer
A benevolent tool in our trade is the refrigerator and freezer. The fridge is extremely useful in extending the longevity of seed and pollen. The trick to successful freezing is to freeze deep (-10 to -40°F/-20 to -35°C) and then keep the seed undisturbed. Hard frozen objects are very fragile. The slightest shock may shatter crucial, delicate cell structures within the seed. Double wrap the seed in paper; little manilla envelopes work great.
I like to do small amounts, in one-time-use packets, to keep waste to a minimum. Then place the wrap into a plastic freezer bag, then place the freezer bag into a plastic tub or tupperware container. Now the seed is ready for the deep-freeze. In the fridge, storing seed in airtight, brown glass jars with a little rice or other non-toxic desiccant seems to work best.
I have had pollen last for years in a deep freeze. It must be frozen immediately after fresh collection from the plant, in as low a humidity as possible (preferably 0%). I like to shake the productive male flowers over a flat and clean piece of glass. The pollen pile is sifted to rid the unwanted plant material from the pure powder.
It is also useful to cut pollen with flour to stretch the amount. A pollen-to-flour ratio of 1:10 or even 1:100 works best. The cut pollen may then be separated into small, one-time-use amounts, stored in a flap of paper and frozen the same way as the seed. The frozen pollen must be applied to the live female flower immediately after thawing to increase viability.
Your friend the freezer
A benevolent tool in our trade is the refrigerator and freezer. The fridge is extremely useful in extending the longevity of seed and pollen. The trick to successful freezing is to freeze deep (-10 to -40°F/-20 to -35°C) and then keep the seed undisturbed. Hard frozen objects are very fragile. The slightest shock may shatter crucial, delicate cell structures within the seed. Double wrap the seed in paper; little manilla envelopes work great.
I like to do small amounts, in one-time-use packets, to keep waste to a minimum. Then place the wrap into a plastic freezer bag, then place the freezer bag into a plastic tub or tupperware container. Now the seed is ready for the deep-freeze. In the fridge, storing seed in airtight, brown glass jars with a little rice or other non-toxic desiccant seems to work best.
I have had pollen last for years in a deep freeze. It must be frozen immediately after fresh collection from the plant, in as low a humidity as possible (preferably 0%). I like to shake the productive male flowers over a flat and clean piece of glass. The pollen pile is sifted to rid the unwanted plant material from the pure powder.
It is also useful to cut pollen with flour to stretch the amount. A pollen-to-flour ratio of 1:10 or even 1:100 works best. The cut pollen may then be separated into small, one-time-use amounts, stored in a flap of paper and frozen the same way as the seed. The frozen pollen must be applied to the live female flower immediately after thawing to increase viability.
Last edited:

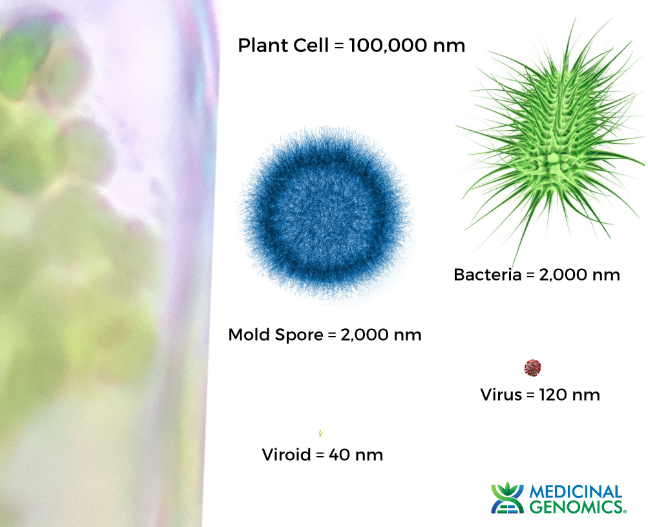



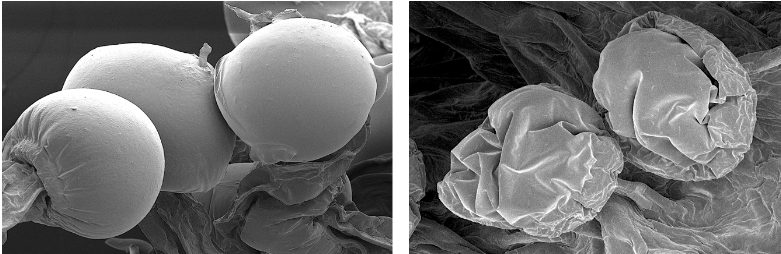
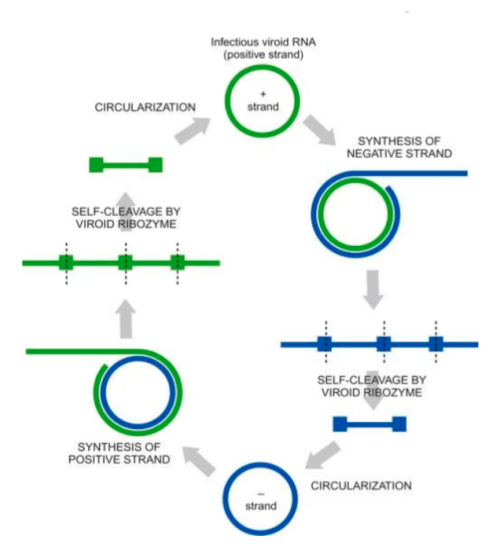
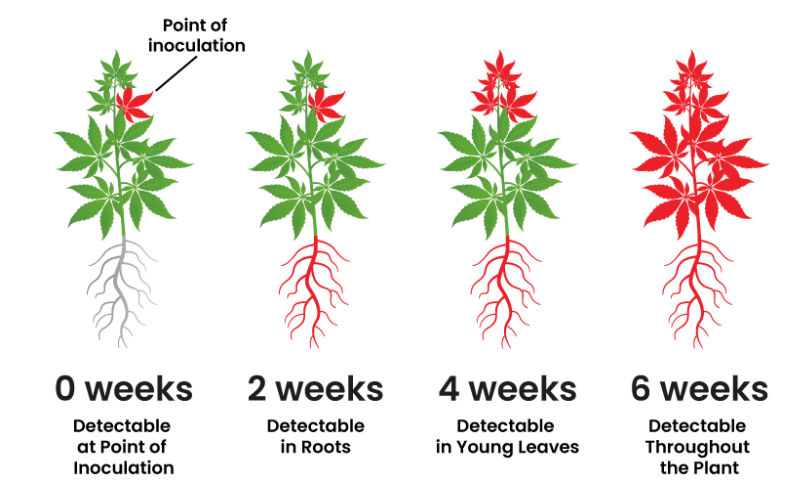
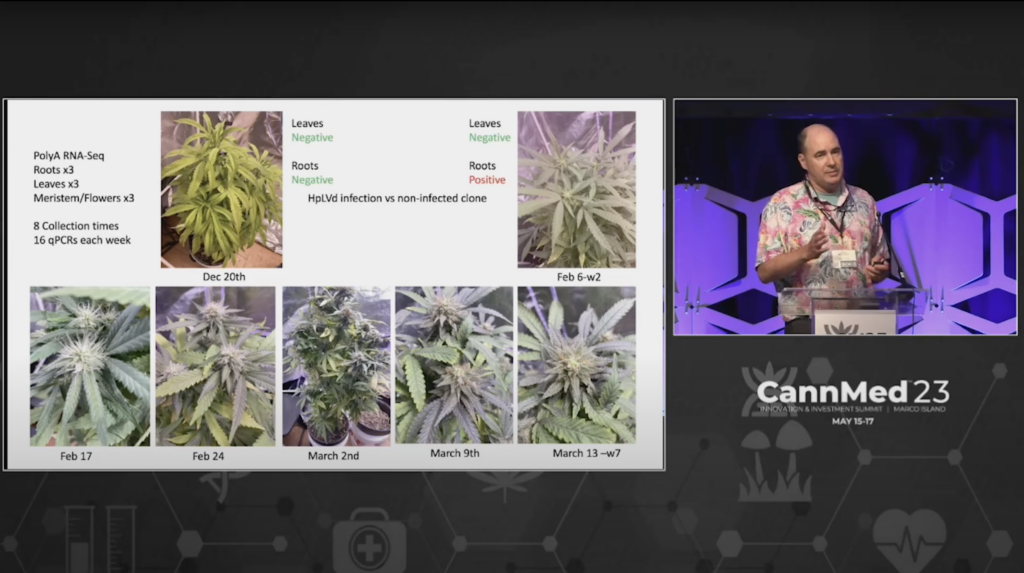 Kevin McKernan shares data that shows HLVd infection stays in Jamaican Lion root at CannMed 23. Click image to view the video.
Kevin McKernan shares data that shows HLVd infection stays in Jamaican Lion root at CannMed 23. Click image to view the video. 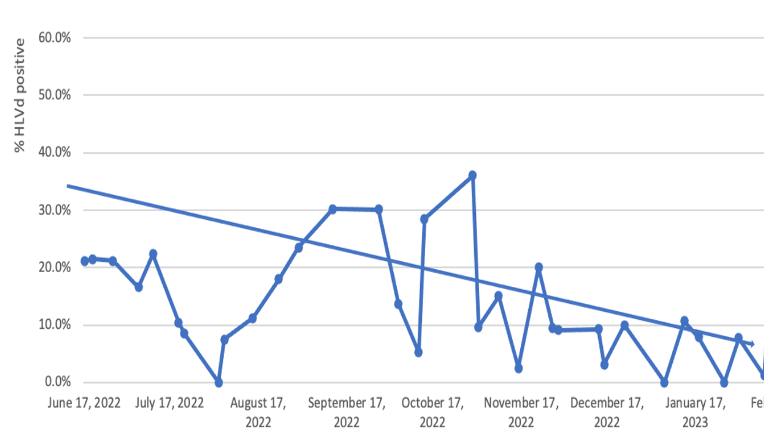




 arduinosoilmosituresensor.ino
arduinosoilmosituresensor.ino