acespicoli
Well-known member
In all cases, all remaining ungerminated seeds have been tested by means of the tetrazolium test (International Seed Testing Association, 1996). Seeds were cut through the embryo prior to imbibition in a 1 % tetrazolium staining solution, stained at 24 °C for one night and seeds were classified as viable when the embryo was well stained and not damaged.
Bio Protoc. 2021 Jan 5; 11(1): e3875.
Published online 2021 Jan 5. doi: 10.21769/BioProtoc.3875
PMCID: PMC7952943
PMID: 33732764
Author information Article notes Copyright and License information Disclaimer
See "Transient expression of the β-glucuronidase gene in Cannabis sativa varieties" in Plant Signal Behav, volume 15, 1780037.
Go to:
Keywords: Cannabis sativa, Rapid germination, Hydrogen peroxide, Seed sterilization, Seedling development
Go to:
Go to:
Results
In this study, we have described a rapid and efficient seed germination protocol for Cannabis sativa. The brief description of this protocol has been reported in Sorokin et al. (2020) . In the current study, we have standardized the optimum concentration of hydrogen peroxide (H2O2) solution media for efficient sterilization and rapid germination. We have tested various concentrations of H2O2 solution as well as sterile water control (H2O, 1% H2O2, 3% H2O2, 5% H2O2, or 10% H2O2) for sterilization and germination efficiency. All three steps of germination (seed sterilization, germination, and seedlings development) were carried out in various concentrations of H2O2 solution and seeds were kept in liquid media for four days. Hydrogen peroxide presents several significant advantages over mercuric chloride or bleach sterilants, which require additional seed washing, and separate germination/seedling development step in Murashige and Skoog (MS) agar medium ( Sorokin et al., 2020 ). The 1% H2O2 solution showed rapid and higher germination than higher H2O2 concentrations solution and water control at day 1 (Figure 1). On day 1, 1% H2O2 solution exhibited 82.5% germination as compared to 22.5% germination for 3% H2O2 group, 17.5% germination for 5% H2O2 group and 47.5% germination in water control group (Figure 1B). Interestingly, 10% H2O2 did not show any germination on day 1 due to its toxic effect (Figure 1). In 1% H2O2 solution, radicle appearance (germination) occurred within 24 h and seedling development (two fully developed cotyledons and two immature true leaves stage) occurred in 72-96 h (Figure 1A). In comparison to previous germination methods which take between 4-7 days for radicle appearance and 5-15 days for seedling development ( Wielgus et al., 2008 and references therein), our germination method resulted in radicle appearance in 1 day and allowed us to obtain cannabis seedlings in a very short period (3-7 days) with minimal efforts (Figures 1-2). Considering the possible toxic effect of H2O2 (since germinated seeds/seedlings stayed continuously in H2O2 solution for 4 days), we have checked further survival of germinated seeds/seedlings on MS media and soil (Figures 2-3). On MS media, 1% H2O2 solution seedlings survived better than other treatments (Figure 2). The water germinated seeds exhibited contamination and did not survive on MS media (Figure 2). Similarly, due to the toxic effect of higher concentration of H2O2, the 10% H2O2 germinated seeds did not survive on MS media (Figure 2). The 1% H2O2 solution seedlings also survived well on soil (Figure 3). Apart from this, we have also tested our method for more than 5-years old cannabis seeds with lower viability, which demonstrated that 1% H2O2 solution medium exhibited a very high germination percentage (~50%) as compared to water control (~10%) (Figure 4). In conclusion, we have developed a rapid and efficient method for C. sativa seed germination under sterile conditions for tissue culture and other sensitive applications.
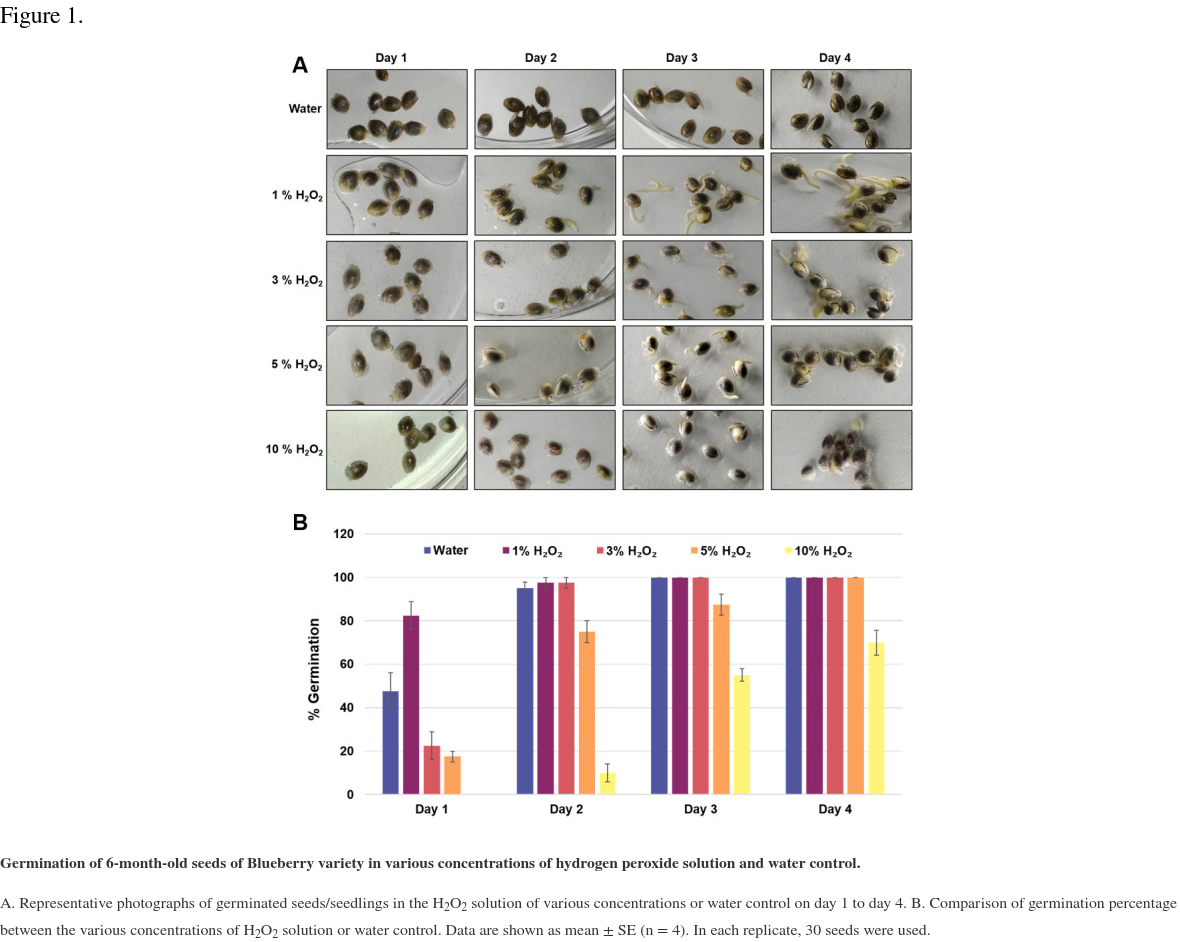
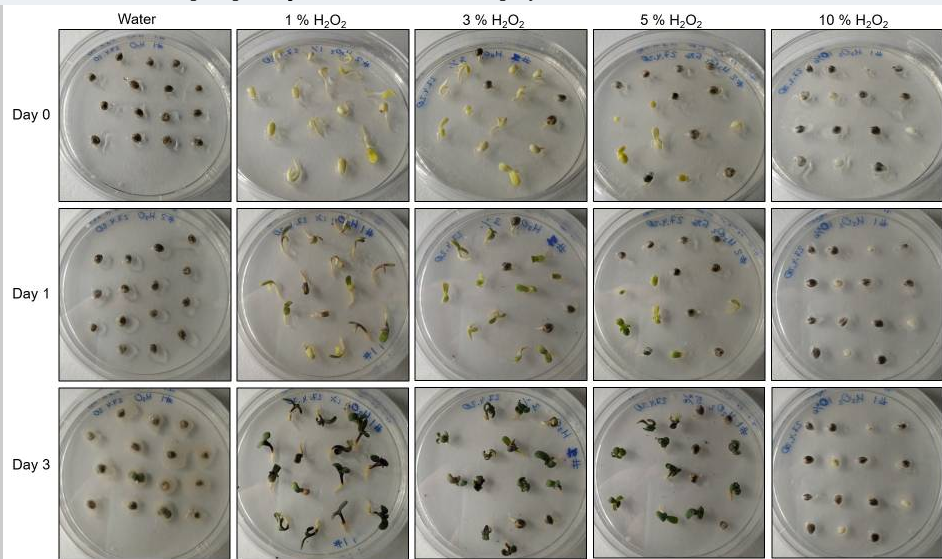
Germination of 6-month-old seeds of Blueberry variety in various concentrations of hydrogen peroxide solution and water control.
A. Representative photographs of germinated seeds/seedlings in the H2O2 solution of various concentrations or water control on day 1 to day 4. B. Comparison of germination percentage between the various concentrations of H2O2 solution or water control. Data are shown as mean ± SE (n = 4). In each replicate, 30 seeds were used.
Representative photographs of growth and survival of H2O2 solutions germinated seeds/seedlings of Blueberry variety on MS media.
The Blueberry variety seeds were soaked in the H2O2 solution (germination solutions) for four days and thereafter, germinated seeds/seedlings were transferred from H2O2 solution to MS medium plates to observe the growth and survival of H2O2 solution germinated seeds/seedlings on MS medium. The photographs were taken at day 0 (just after transfer to MS medium plates), day 1 (after 24 h of the transfer to MS medium plates), and day 3 (after 72 h of the transfer to MS medium plates) on MS media.

Representative photograph of Blueberry variety young plantlet growing in soil (Pro-Mix HP Mycorrhizae Growing Medium).
The Blueberry variety seeds were soaked in the H2O2 solution (germination solutions) for four days and thereafter, germinated seeds/seedlings were transferred from H2O2 solution to soil pot (Pro-Mix HP Mycorrhizae Growing Medium) to observe the growth and survival of H2O2 solution germinated seeds/seedlings on soil. The photographs were taken on day 12.
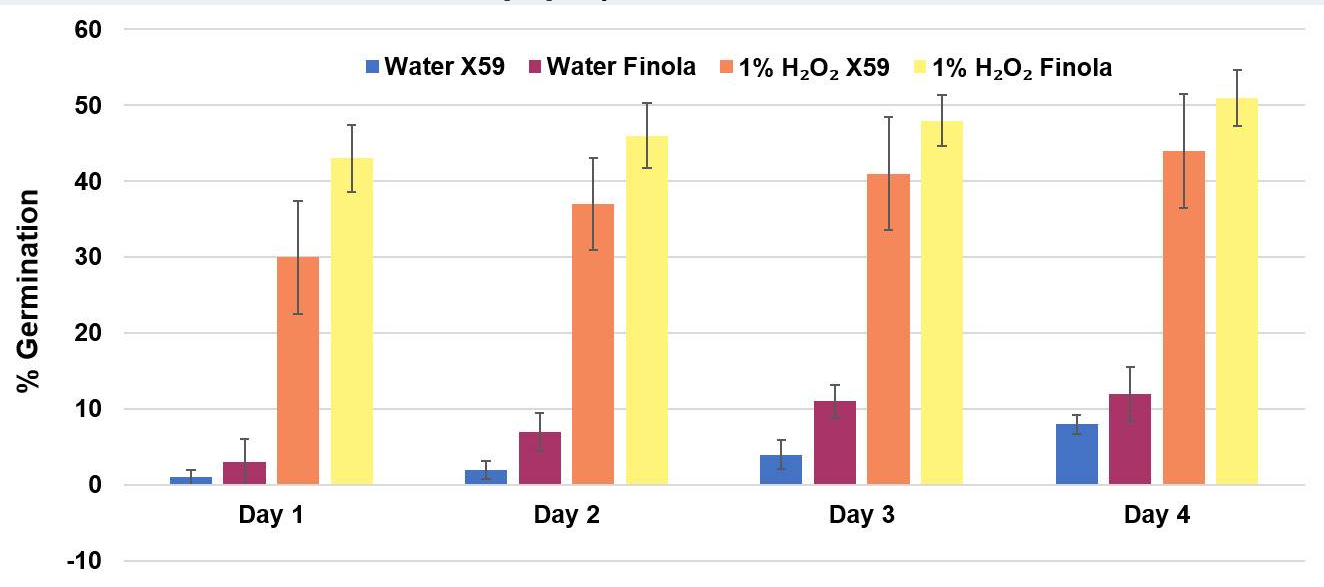
Germination of 5-years old seeds of Finola and X59 varieties in 1% hydrogen peroxide solution and water control.
Comparison of germination percentage between 1% H2O2 solution media and water control. Data are shown as mean ± SE (n = 5). In each replicate, around 30 seeds were used.
Go to:
Go to:
2. Gaudet D., Yadav N. S., Sorokin A., Bilichak A. and Kovalchuk I.(2020). Development and optimization of a germination assay and long-term storage for Cannabis sativa pollen . Plants 9: 665. [PMC free article] [PubMed] [Google Scholar]
3. Sorokin A., Yadav N. S., Gaudet D. and Kovalchuk I.(2020). Transient expression of the β-glucuronidase gene in Cannabis sativa varieties . Plant Signal Behav 15(8): 1780037. [PMC free article] [PubMed] [Google Scholar]
4. Wielgus K., Luwanska A., Lassocinski W. and Kaczmarek Z.(2008). Estimation of Cannabis sativa L. tissue culture conditions essential for callus induction and plant regeneration . J Nat Fibers 5: 199-207. [Google Scholar]

Sanyo MLR-350H Pervious Panasonic MLR-352 Previous Sanyo MLR-351
Modeling and optimizing in vitro seed germination of industrial hemp (Cannabis sativa L.)
Author: Mohsen Hesami, Marco Pepe, Adrian Scott Monthony, Austin Baiton, Andrew Maxwell Phineas Jones Source: Industrial crops and products 2021 v.170 pp. 113753 ISSN: 0926-6690 Subject: Cannabis sativa, culture media, genotype, glucose, hemp, juveniles, neural networks, prediction, seed germination, sucrose, walnuts Abstract: In vitro seed germination of cannabis as the first physiological stage in the plant life cycle is not only important for studying factors affecting cultivation conditions but also crucial for obtaining juvenile tissue as a potential explant for different in vitro procedures. On the other hand, in vitro seed germination is a multi-variable biological process that can be influenced by genetic (genotype) and physical factors (medium composition and environmental conditions). Therefore, a powerful mathematical methodology such as artificial neural networks (ANNs) is well suited to analyze the data and optimize the conditions this complex system. The current study was aimed to evaluate the effect of different types and concentrations of carbohydrate sources (sucrose and glucose) as well as different strengths of DKW (Driver and Kuniyaki Walnut) and mMS (Murashige and Skoog Medium, Van der Salm modification) media on seed germination indices as well as morphological features of in vitro-grown cannabis seedlings by using three ANNs including multilayer perceptron (MLP), radial basis function (RBF), and generalized regression neural network (GRNN). The GRNN model displayed higher predictive accuracy (r²>0.70) in both training and testing sets for all germination indices and morphological traits in comparison to RBF or MLP. Moreover, non-dominated sorting genetic algorithm-II (NSGA-II) was subjected to the GRNN to find the optimal type and level of media and carbohydrate source for obtaining the best seed germination indices (germination rate and mean germination time). According to the optimization process, 0.43 strength mMS medium supplemented with 2.3 % sucrose would result in the best outcomes. This result showed that a moderate level of salts existing in culture media (0.43 strength of mMS medium) supplemented with a moderate level of sucrose (2.3 %) can improve in vitro seed germination of hemp. The results of a validation experiment revealed that there was a negligible difference between the experimental data and the optimized result. Therefore, GRNN-NSGA-II provided an accurate prediction of seed germination and can likely be employed to optimize different factors involved in in vitro culture of this multi-purpose crop. Agid: 7423019
Development and Standardization of Rapid and Efficient Seed Germination Protocol for Cannabis sativa - PMC
Cannabis seed germination is an important process for growers and researchers alike. Many biotechnological applications require a reliable sterile method for seed germination. This protocol outlines a seed germination procedure for Cannabis sativa ...
 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov
Bio Protoc. 2021 Jan 5; 11(1): e3875.Development and Standardization of Rapid and Efficient Seed Germination Protocol for Cannabis sativa - PMC
Cannabis seed germination is an important process for growers and researchers alike. Many biotechnological applications require a reliable sterile method for seed germination. This protocol outlines a seed germination procedure for Cannabis sativa ...
Published online 2021 Jan 5. doi: 10.21769/BioProtoc.3875
PMCID: PMC7952943
PMID: 33732764
Development and Standardization of Rapid and Efficient Seed Germination Protocol for Cannabis sativa
Aleksei Sorokin,# Narendra Singh Yadav,# Daniel Gaudet, and Igor Kovalchuk*Author information Article notes Copyright and License information Disclaimer
See "Transient expression of the β-glucuronidase gene in Cannabis sativa varieties" in Plant Signal Behav, volume 15, 1780037.
Go to:
Abstract
Cannabis seed germination is an important process for growers and researchers alike. Many biotechnological applications require a reliable sterile method for seed germination. This protocol outlines a seed germination procedure for Cannabis sativa using a hydrogen peroxide (H2O2) solution as liquid germination media. In this protocol, all three steps including seed sterilization, germination, and seedlings development were carried out in an H2O2 solution of different concentrations; 1% H2O2 solution showed the fastest and the most efficient germination. This protocol also exhibited high germination efficiency for very old cannabis seeds with lower viability. Overall, this protocol demonstrates superior germination compared to water control and reduces the risk of contamination, making it suitable for tissue culture and other sensitive applications.Keywords: Cannabis sativa, Rapid germination, Hydrogen peroxide, Seed sterilization, Seedling development
Go to:
Background
Cannabis sativa, otherwise known as marijuana or hemp, is an annual primarily dioecious flowering plant in which male/female sex is determined by heteromorphic chromosomes (X and Y) ( Gaudet et al., 2020 ). Cannabis is grown for a variety of agricultural uses; nearly all parts of cannabis plant are used, seeds for food, stem for fiber, and flowers/leaves for medicine. Flowers produce a mix of cannabinoids and aromatic compounds valued for their therapeutic and recreational effects ( Chandra et al., 2017 ). Cannabis plants are propagated either clonally through cuttings or via seed germination. Seed germination is very important for researchers, breeders, and growers alike, especially since seeds from elite cultivars can be very expensive and valuable. Additionally, older seeds may have a reduced germination rate while bacterial and fungal contamination can compromise germination, especially when seeds are germinated for tissue culture propagation. To address these issues, we have developed a rapid, sterile, and efficient seed germination protocol using a 1% hydrogen peroxide (H2O2) solution. In this protocol, all three steps including seed sterilization, germination, and seedlings development were carried out in a 1% H2O2 solution. This presents a significant advantage over other sterilants, such as mercuric chloride or bleach, which require additional washing of seeds and a separate germination step on MS solid medium. Our protocol resulted in faster germination and increased seed germination percentage as compared to water control, with no bacterial or fungal contamination, making it suitable for tissue culture and other sensitive applications. In comparison to previous germination methods which take between 4-7 days for radicle appearance and 5-15 days for seedling development ( Wielgus et al., 2008 and references therein), our germination method resulted in radicle appearance in 1 day and allowed us to obtain cannabis seedlings in a very short period (3-7 days) with minimal efforts. This protocol is also very efficient for germination of very old cannabis seeds with lower viability.Go to:
Materials and Reagents
- Biological materials
- Cannabis sativa (Finola, X59, and Blueberry varieties) seeds
All seeds were harvested in our laboratory. Blueberry seeds were not older than 6 months, when employed in the experiments. Finola and X59 seeds were more than 5 years old.
- Cannabis sativa (Finola, X59, and Blueberry varieties) seeds
- Chemicals
- Hydrogen Peroxide 30% (Merck®, catalog number: 1072091000)
- Murashige & Skoog Basal Medium with Vitamins (PhytoTechnology Laboratories®, catalog number: M519)
- Sucrose (Sigma-Aldrich, catalog number: S0389)
- MES (Sigma-Aldrich, catalog number: M3671)
- Agar type E (Sigma-Aldrich, catalog number: A4675)
- MS solid media (1 L) (see Recipes)
- Plasticware
- Sterile empty 100 x 15 mm Petri plates (VWR International, catalog number: 25384-342)
- Sterile disposable 15 or 50 ml screw-cap centrifuge tubes (BD, FalconTM, catalog number: 352070)
Equipment
- Laminar flow hood (Microzone Bio Klone 2, catalog number: 30193-086)
- pH meter (Corning Model 430, catalog number: 475303)
- Sterile forceps and scalpel (sterilized by heat treatment using a Bunsen burner)
- Growth chamber (Sanyo MLR-350, catalog number: 859-600-06): 24 °C, 18 h light/6 h dark cycle, light intensity 200 μmol·m-2·sec-1
- Pro-Mix HP Mycorrhizae Growing Medium (Pro-Mix, catalog number: 20381RG)
Procedure
Seed germination assay- Soak seeds overnight in various concentrations of hydrogen peroxide solution (liquid germination media or germination solutions) as well as in sterile water control (H2O, 1% H2O2, 3% H2O2, 5% H2O2, or 10% H2O2) in 15 or 50 ml screw-cap (Falcon tube). Falcon tubes with submerged seeds in various germination solutions were kept in the dark at room temperature.
- Next day, record the percentage of germinated seeds in germination solution (appearance of radicle is considered as germination event) and add fresh respective germination solution after removal of old solution simply by pouring out.
- Keep seeds soaked in the same solution for 3 more days in the dark at room temperature and record the percentage of germinated seeds every day.
- Thereafter, germinated seeds/seedlings were transferred with or without seed coats from H2O2 solution to MS medium plates to observe the growth of H2O2 solution-germinated seeds/seedlings on MS medium. To transfer, first germinated seeds/seedlings were poured together with H2O2 solution from the Falcon tube to the empty petri plate. Then seedlings were transferred to sterile paper by using forceps to remove excess H2O2 solution. Finally, the germinated seeds/seedlings were transferred to MS media plate by using forceps. The whole transfer process has been carried out in the laminar flow hood.
- Parafilm sealed MS medium plates with germinated seeds/seedlings are then transferred to the growth chamber (24 °C, 18 h light/6 h dark cycle and light intensity 200 μmol·m-2·sec-1) for 3 days to observe the growth and survival of H2O2 solution germinated seeds/seedlings on MS medium.
- The H2O2 solution-germinated seeds/seedlings growth was also observed in soil. Pro-Mix HP Mycorrhizae Growing Medium used for soil experiment. The cannabis seeds were soaked in the H2O2 solution (germination solutions) for four days and thereafter, germinated seeds/seedlings were transferred from H2O2 solution to soil pot (Pro-Mix HP Mycorrhizae Growing Medium) to observe the growth and survival of H2O2 solution germinated seeds/seedlings on soil. The soil pots were transferred to the growth chamber (24 °C, 18 h light/6 h dark cycle and light intensity 200 μmol·m-2·sec-1). The photographs were taken on day 12.
Data analysis
Mean seed germination percentage under various concentrations of H2O2 solution as well as water control were calculated in an excel sheet. Data were shown as mean ± SE.Results
In this study, we have described a rapid and efficient seed germination protocol for Cannabis sativa. The brief description of this protocol has been reported in Sorokin et al. (2020) . In the current study, we have standardized the optimum concentration of hydrogen peroxide (H2O2) solution media for efficient sterilization and rapid germination. We have tested various concentrations of H2O2 solution as well as sterile water control (H2O, 1% H2O2, 3% H2O2, 5% H2O2, or 10% H2O2) for sterilization and germination efficiency. All three steps of germination (seed sterilization, germination, and seedlings development) were carried out in various concentrations of H2O2 solution and seeds were kept in liquid media for four days. Hydrogen peroxide presents several significant advantages over mercuric chloride or bleach sterilants, which require additional seed washing, and separate germination/seedling development step in Murashige and Skoog (MS) agar medium ( Sorokin et al., 2020 ). The 1% H2O2 solution showed rapid and higher germination than higher H2O2 concentrations solution and water control at day 1 (Figure 1). On day 1, 1% H2O2 solution exhibited 82.5% germination as compared to 22.5% germination for 3% H2O2 group, 17.5% germination for 5% H2O2 group and 47.5% germination in water control group (Figure 1B). Interestingly, 10% H2O2 did not show any germination on day 1 due to its toxic effect (Figure 1). In 1% H2O2 solution, radicle appearance (germination) occurred within 24 h and seedling development (two fully developed cotyledons and two immature true leaves stage) occurred in 72-96 h (Figure 1A). In comparison to previous germination methods which take between 4-7 days for radicle appearance and 5-15 days for seedling development ( Wielgus et al., 2008 and references therein), our germination method resulted in radicle appearance in 1 day and allowed us to obtain cannabis seedlings in a very short period (3-7 days) with minimal efforts (Figures 1-2). Considering the possible toxic effect of H2O2 (since germinated seeds/seedlings stayed continuously in H2O2 solution for 4 days), we have checked further survival of germinated seeds/seedlings on MS media and soil (Figures 2-3). On MS media, 1% H2O2 solution seedlings survived better than other treatments (Figure 2). The water germinated seeds exhibited contamination and did not survive on MS media (Figure 2). Similarly, due to the toxic effect of higher concentration of H2O2, the 10% H2O2 germinated seeds did not survive on MS media (Figure 2). The 1% H2O2 solution seedlings also survived well on soil (Figure 3). Apart from this, we have also tested our method for more than 5-years old cannabis seeds with lower viability, which demonstrated that 1% H2O2 solution medium exhibited a very high germination percentage (~50%) as compared to water control (~10%) (Figure 4). In conclusion, we have developed a rapid and efficient method for C. sativa seed germination under sterile conditions for tissue culture and other sensitive applications.
Germination of 6-month-old seeds of Blueberry variety in various concentrations of hydrogen peroxide solution and water control.
A. Representative photographs of germinated seeds/seedlings in the H2O2 solution of various concentrations or water control on day 1 to day 4. B. Comparison of germination percentage between the various concentrations of H2O2 solution or water control. Data are shown as mean ± SE (n = 4). In each replicate, 30 seeds were used.
Representative photographs of growth and survival of H2O2 solutions germinated seeds/seedlings of Blueberry variety on MS media.
The Blueberry variety seeds were soaked in the H2O2 solution (germination solutions) for four days and thereafter, germinated seeds/seedlings were transferred from H2O2 solution to MS medium plates to observe the growth and survival of H2O2 solution germinated seeds/seedlings on MS medium. The photographs were taken at day 0 (just after transfer to MS medium plates), day 1 (after 24 h of the transfer to MS medium plates), and day 3 (after 72 h of the transfer to MS medium plates) on MS media.
Representative photograph of Blueberry variety young plantlet growing in soil (Pro-Mix HP Mycorrhizae Growing Medium).
The Blueberry variety seeds were soaked in the H2O2 solution (germination solutions) for four days and thereafter, germinated seeds/seedlings were transferred from H2O2 solution to soil pot (Pro-Mix HP Mycorrhizae Growing Medium) to observe the growth and survival of H2O2 solution germinated seeds/seedlings on soil. The photographs were taken on day 12.
Germination of 5-years old seeds of Finola and X59 varieties in 1% hydrogen peroxide solution and water control.
Comparison of germination percentage between 1% H2O2 solution media and water control. Data are shown as mean ± SE (n = 5). In each replicate, around 30 seeds were used.
Go to:
Recipes
- MS solid media (1 L)
4.43 g Murashige & Skoog Basal Medium with Vitamins
500 mg MES
30 g Sucrose
8 g Agar
Adjust pH to 5.7 with KOH and sterilize by autoclaving at 121 °C for 40 min. 25 ml of MS media on each Petri plate.
Acknowledgments
This protocol is derived from Sorokin et al. (2020). We thank the Natural Sciences and Engineering Research Council of Canada (NSERC) and MITACS for funding our work.Go to:
References
1. Chandra S., Lata H. and ElSohly M. A.(2017). Cannabis sativa L.-botany and biotechnology. Chandra, S., Lata, H. and ElSohly, M. A.(Eds.). Springer International Publishing: Cham, Switzerland. ISBN: 9783319545639. [Google Scholar]2. Gaudet D., Yadav N. S., Sorokin A., Bilichak A. and Kovalchuk I.(2020). Development and optimization of a germination assay and long-term storage for Cannabis sativa pollen . Plants 9: 665. [PMC free article] [PubMed] [Google Scholar]
3. Sorokin A., Yadav N. S., Gaudet D. and Kovalchuk I.(2020). Transient expression of the β-glucuronidase gene in Cannabis sativa varieties . Plant Signal Behav 15(8): 1780037. [PMC free article] [PubMed] [Google Scholar]
4. Wielgus K., Luwanska A., Lassocinski W. and Kaczmarek Z.(2008). Estimation of Cannabis sativa L. tissue culture conditions essential for callus induction and plant regeneration . J Nat Fibers 5: 199-207. [Google Scholar]
Sanyo MLR-350H Pervious Panasonic MLR-352 Previous Sanyo MLR-351
Last edited: