@ highonmt:
One thing I forgot to mention: the main reason using an absorption spectra of Chl or accessory pigments is not sound in terms of light for photosynthesis (besides issues of in vitro vs in vivo and spectrophotometer vs integrating sphere/spectroradiometer and Pn/Chl fluorometer chambers) is a surprising amount of photons absorbed are not used for photosynthesis, much is lost as heat from the leaf. That is why using action spectra of photosynthesis (ASP) and relative quantum yield (QY), either alone or together, is much better than using the absorption spectra of Chl and some carotinoids as you posted above (as commonly found in vitro with a spectrophotometer). Out of all three, using a relative quantum yield curve is the best choice.
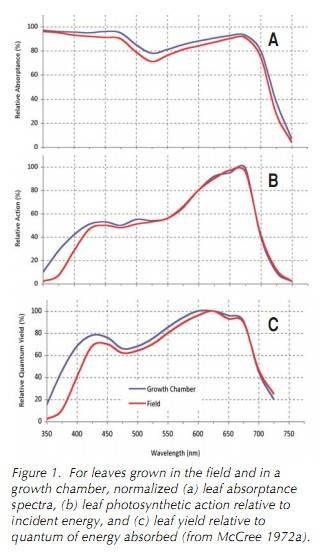
I have many papers on this topic, but below is a good intro and has good graphs showing differences in (normalized) spectra between "(a) leaf absorptance spectra, (b) leaf photosynthetic action relative to incident energy, and (c) leaf yield relative to quantum of energy absorbed (from McCree 1972a)". This paper was written by a plant physiologist I have spoken to many times, he works at Li-cor, the brand of quantum sensor I use to find irradiance in light studies and when setting grows rooms (i.e., umol/area/second).
That paper also discusses usage of an integrating sphere, i.e., absorbency, reflectance and transmittance. Fwiw, I plan on buying an integrating sphere/spectroradiometer to create action spectras of photosynthesis for cannabis, as well as creating quantum yield curves for cannabis after I buy a Pn/Chl fluorometer chamber. I still have much to learn about making ASP's and QY curves, but it's something I really want to accomplish for the betterment of the science regarding cannabis.
One thing I forgot to mention: the main reason using an absorption spectra of Chl or accessory pigments is not sound in terms of light for photosynthesis (besides issues of in vitro vs in vivo and spectrophotometer vs integrating sphere/spectroradiometer and Pn/Chl fluorometer chambers) is a surprising amount of photons absorbed are not used for photosynthesis, much is lost as heat from the leaf. That is why using action spectra of photosynthesis (ASP) and relative quantum yield (QY), either alone or together, is much better than using the absorption spectra of Chl and some carotinoids as you posted above (as commonly found in vitro with a spectrophotometer). Out of all three, using a relative quantum yield curve is the best choice.
I have many papers on this topic, but below is a good intro and has good graphs showing differences in (normalized) spectra between "(a) leaf absorptance spectra, (b) leaf photosynthetic action relative to incident energy, and (c) leaf yield relative to quantum of energy absorbed (from McCree 1972a)". This paper was written by a plant physiologist I have spoken to many times, he works at Li-cor, the brand of quantum sensor I use to find irradiance in light studies and when setting grows rooms (i.e., umol/area/second).
That paper also discusses usage of an integrating sphere, i.e., absorbency, reflectance and transmittance. Fwiw, I plan on buying an integrating sphere/spectroradiometer to create action spectras of photosynthesis for cannabis, as well as creating quantum yield curves for cannabis after I buy a Pn/Chl fluorometer chamber. I still have much to learn about making ASP's and QY curves, but it's something I really want to accomplish for the betterment of the science regarding cannabis.
"Comparison of Quantum Sensors with Different Spectral Sensitivities"
http://www.licor.com/env/pdf/light/TechNote126.pdf
http://www.licor.com/env/pdf/light/TechNote126.pdf


 Thanks
Thanks
