GRINKEEEPER
Member
I love this kind of thread where there is two sides to the truth.... keep it alive
Around 50% of green light is reflected. The rest is used - got it
No, not "more than twice" the rate of Pn verses blue and red. But green light should be included in all light sources, there are various photo-reactions that take place thanks to green light (like increased Co2 fixation in lower section of leaf, reduced stretching, bigger leafs, etc.); incl. increased leaf and whole canopy Pn.But are you saying it drives photosynthesis more than twice as good as blue and red, and should be used in a LED array regardless of the 50% loss by reflection?
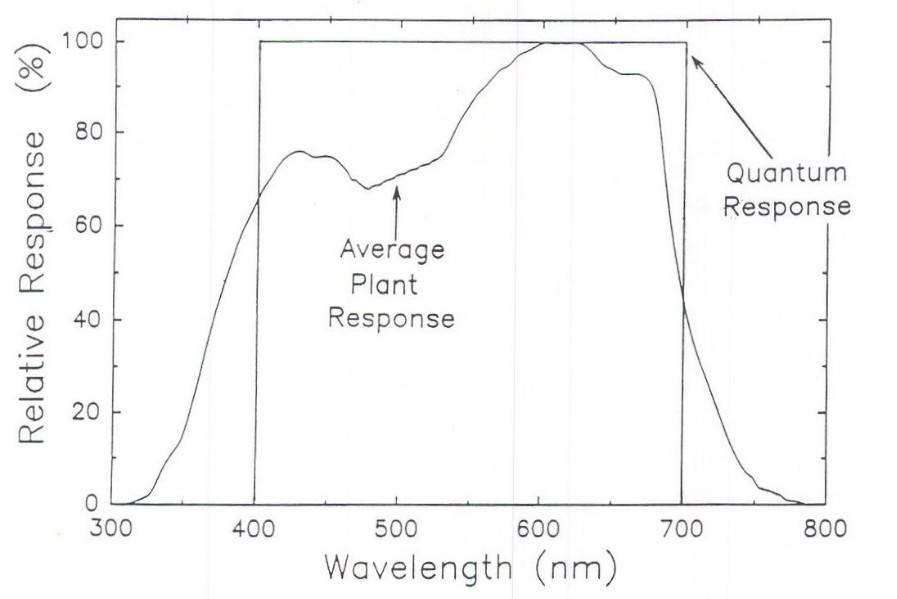
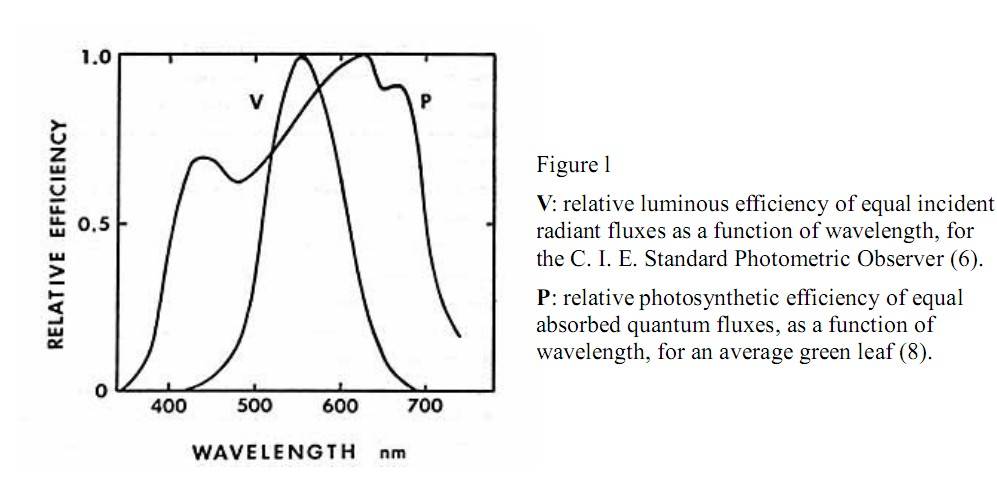
Here is the ASP of PAR range light from K.McCree (i.e., basically how plants use PAR range light for Pn) verses the luminous efficiency of PAR range light (i.e., how human eyes use PAR range light to see). The inset line (V) is how lumen and lux meters are weighted, i.e., they over-report green light, that is why they are not good for measuring light for plants:Fig. 2. The quantum (PPF) response when all photons are weighted equally between 400 and 700 nm; and the relative quantum efficiency curve as determined by the average plant response for photosynthesis (from McCree, 1972a). The quantum response overestimates the photosynthetic value of photons between 400 and about 550 nm, but underestimates the photosynthetic value of photons below 400 and above 700 nm.
Here are some studies and refs:In these reports the effects of combinatorial red, blue, and green (RB+G) light treatments on leaf growth and stomatal conductance in lettuce were compared to red and blue (RB) alone (Kim et al., 2004a, b). Green light supplied by green fluorescent lamps was added to a background of red and blue LED light. There was very little (if any) far-red light which is important for discounting potential phytochrome interpretations. The authors discovered that lettuce plants grown in RB+G treatments displayed leaves with larger specific leaf area and less thickness compared with RB alone (Kim et al., 2004a). Also, plants grown under RB treatments demonstrated higher stomatal conductance when compared with those under RB+G, with the lowest stomatal conductance reported in plants grown under green fluorescent lamps alone (Kim et al., 2004b). In addition, while stomatal conductance was greater in cool white fluorescent treatments than in RB+G, the dry mass of the plants was greater in RB+G implying the weaker stomatal conductance did not negatively affect carbon assimilation (Kim et al., 2004b). Plant dry mass was greatest under RB+G treatments (where 24% of the spectrum was broadband green light) when compared with RB, the opposite of the effects noted by Went (1957; Fig. 2). However, these results do agree with previous findings that plants grown in RB+G treatments displayed larger specific leaf areas than those grown under RB treatments (Kim et al., 2004a). These experiments demonstrate that supplemental green affects plant physiology in conditions where red and blue systems are saturated. It remains to be seen if these effects are cry-dependent or cry-independent, as they were performed in species where photoreceptor mutants are not yet available.

mr. mustard said:Green light isn't worth a fart in a jar if you're a plant... that's why they're green
that there is bullshit
Kopite

yep, plants use green light just fine- why wouldnt they when it's such a large part of the sunlight spectrum.

I'm eagerly awaiting an explanation as to why plants without green light do just fine.
I was happy to stay out of the Breeder's Forum when I realized what was to be found for "knowledge".
Just say the word and I'll stay out of this one too.
I'm really curious to what could be the magic you get from green.
So what makes the advantage twice as big as blue or red, and there by better.. Anyone? Spurr??

This news about green light and the plants using is starting to worry me!..I always thought green light was unusable to the plants and thats why they sell green headlamps, flashlight, work-lights, etc for working in the grow room during the dark cycle.
Do you think the green light given off from a Bluelab Guardian PH/Temp/EC Monitor is affecting my dark cycle? Is my green LED work-light I use affecting my dark cycle? I need to know this as I run the Guardian 24/7 and I go in during the dark cycle sometimes with the work-light. Thanks!
SS
afaik, certain colours such as red reach a saturation point beyond which they just get converted to heat in the leaf rather than sugar/starch. including green gives a whole new 'dose' of light that the plant can efficiently use when it may not be able to use more red. also because green gets partly reflected by leaves it bounces around under the canopy and gets used down there where red and blue tend not to reach...
VG

couple more links, spurr may have some full text of the above one
http://wwwsoc.nii.ac.jp/jsac/analsci/special/030.pdf
http://www.tutorvista.com/content/b...synthesis/photosynthesis-external-factors.php
Math-durbators will rise and follow him! The rest will need actual proof.
Here's a tent full of plants grown entirely with 300 watts of LEDs at day 42 of 12/12. They stand 36" tall and the central colas are 18" by 3 1/2 inches. In fact the only thing hold back their height was the top of the tent. It's nice to read and try to understand how things will affect growth and maturation, but in the end experimentation will prove theories right or wrong.

If you like HID's fine! But don't try and and BS others into following you in order to stroke your ego, it's just not right.
I would venture a guess that plants wouldn't store green light as it would be a huge amount of heat... the less voluminous light would be better for storage and adaptation for usage thereof.
Yup, agree 100%. So I see your Kopite reincarnated, now I understand why you dislike me. (re: 24 hour light thread).

I would venture a guess that plants wouldn't store green light as it would be a huge amount of heat... the less voluminous light would be better for storage and adaptation for usage thereof.
I'll leave you to inundate the public with the saving grace of green light.
Meanwhile I'll be out engineering the next green grow bulb.
I believe the lettuce text was a joke right?Basically yes, but not all photons (incl. blue-green-red) that are absorbed are used for Pn (rate of photosynthesis), much is lost as heat from the leaf (e.g., at light saturation).
No, not "more than twice" the rate of Pn verses blue and red. But green light should be included in all light sources, there are various photo-reactions that take place thanks to green light (like increased Co2 fixation in lower section of leaf, reduced stretching, bigger leafs, etc.); incl. increased leaf and whole canopy Pn.
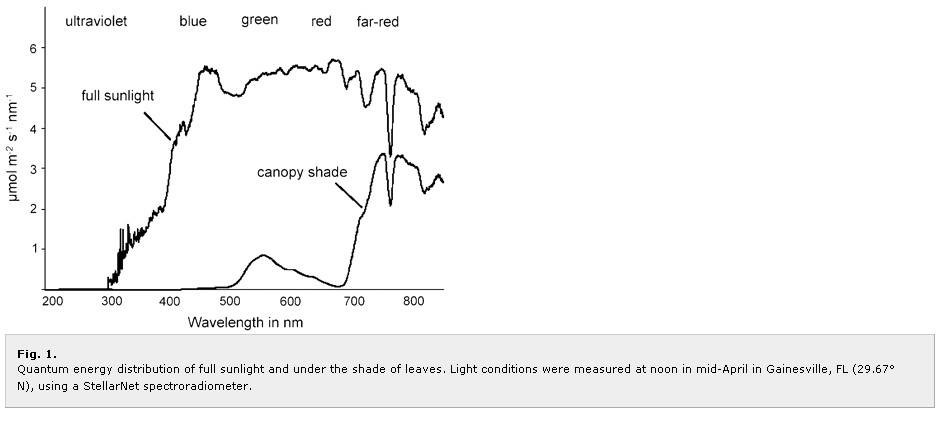
Green light, like far-red light, has higher degree of transmittance through leafs than does blue and red. Also, due to green light reflectance of leafs, more green light reaches intracanopy than does blue and red. Thus, green light is helpful/important in terms of irradiation of lower/intra-canopy to increase "whole canopy" photosynthesis, as well as increase lower/intra-canopy bud sizes.
So, we can see that green light increases Pn at the canopy and whole canopy too. Green light drives Pn under lower irradiance at canopy, but under high irradiance green light can drive Pn very well by acting upon lower chloroplasts in canopy leafs. And by increased irradiance intracanopy green light increases whole canopy Pn.
The following graph shows PAR range PPFD (photosynthetic photon flux density) under full sun and intracanopy; this illustrates how green light better irradiates lower/intra-canopy than does blue and red light, especially under a "closed canopy" like SCROG, tight SOG, etc:
Granted, green light does have some unwelcome effects, such as reduced stomatal openness (that said, red light closes stoma more than green light), as well as hindrance of PhyB flowering response, etc.
In terms of rate of photosynthesis (Pn), the levels of blue/green/red are less important than is the overall irradiance (i.e., PPFD). That is, as long as there is sufficient amounts of each light type, there should be about 10-20% green light, 40-50% red light and 30-40% blue light. In cannabis plants the leafs supply most of the assimilate (from Pn) to the bud it is attached, just like cucumbers as cited by A. Tikhomirov. Not only that, but the buds themselves photosynthesize, that fact is little realized by cannabis growers, but it is a fact none the less.
In the graph below the "quantum response" is what unweighted PPFD (Photosynthetic Photon Flux Density) looks like. PPFD is what a quantum sensor measures; ideally it's 100% unweighted. To weight PPFD to create the lamps' "Quantum Flux Density" we would use the "Average Plant Response" in the figure below, which is really the "Action Spectra of Photosynthesis" from K.McCree. However, as I wrote, we should realize that green light plays a bigger role in Pn (esp. whole canopy verses single leaf) than is shown in the Action Spectra of Photosynthesis (ASP). When K.McCree's ASP was made he used monochromatic lighting, thus his work didn't show the higher Pn from green light (in a single leaf) once blue and red light saturate upper chloroplasts in a leaf.
Under high irradiance the green light in the figure below would be higher (i.e., higher quantum (Pn) efficiency; re: "quantum yield"):
"EFFECTS OF RADIATION QUALITY, INTENSITY, AND DURATION ON PHOTOSYNTHESIS AND GROWTH"
by Bruce Bugbee
International Lighting in Controlled Environments Work
http://www.controlledenvironments.org/Light1994Conf/1_5_Bugbee/Bugbee text.htm
Here is the ASP of PAR range light from K.McCree (i.e., basically how plants use PAR range light for Pn) verses the luminous efficiency of PAR range light (i.e., how human eyes use PAR range light to see). The inset line (V) is how lumen and lux meters are weighted, i.e., they over-report green light, that is why they are not good for measuring light for plants:
Green light and LEDs:
According to Folta and Maruhnich (2007), re: the paper by Kim, et al., (2004), titled "Green light supplementation for enhanced lettuce growth under red and blue light-emitting diodes":
Here are some studies and refs:
1. "Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves are Green"
by Ichiro Terashima, Takashi Fujita, Takeshi Inoue, Wah Soon Chowand Riichi Oguchi
Plant and Cell Physiology 2009 50(4):684-697
(full text) http://pcp.oxfordjournals.org/content/50/4/684.abstract
2. "Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement"
by J.N. Nishio
Plant, Cell and Enviromment (2000) 23, 539-548
(upload to this post)
3. "Photosynthetic Research in Plant Science"
by Ayumi Tanaka and Amane Makino
Plant Cell Physiol. 2009 April; 50(4): 681–683.
(uploaded to this post)
4. "Green Light Drives CO2 Fixation Deep within Leaves"
Jindong Sun, John N. Nishio and Thomas C. Vogelmann
Plant Cell Physiol (1998) 39 (10): 1020-1026.
http://pcp.oxfordjournals.org/content/39/10/1020.short
6. "Green light supplementation for enhanced lettuce growth under red and blue light-emitting diodes"
H-H. Kim, G. Goins, R. Wheeler, J. Sager
[SIZE=-1]HortScience 39: 1617-1622 (2004)[/SIZE]
7. "Green light: a signal to slow down or stop"
Kevin M. Folta, Stefanie A. Maruhnich
J. Exp. Bot. (2007) 58 (12): 3099-3111.
8. "SPECTRAL COMPOSITION OF LIGHT AND GROWING OF PLANTS IN CONTROLLED ENVIRONMENTS"
by Alexander A. Tikhomirov
International Lighting in Controlled Environments Works
T.W.Tibbitts (editor) 1994 NASA-CP-95-3309
http://www.controlledenvironments.org/Light1994Conf/1_3_Tikhomirov/Tikhomirov text.htm
9. Action spectra for photosynthesis in higher plants.
by Katsumi Inada
Plant and Cell Physiology, vol. 17, no. 2, pp. 355-365 (1976)
10. "The action spectrum, absorptance and quantum yield of photosynthesis in crop plants"
K.J. McCree
Agric.Meteorol., vol. 9, pp. 191-216 (1972)
11. "Test of current definitions of photosynthetically active radiation against actual leaf photosynthesis data"
K.J. McCree
Agric. Meteorol., vol. 10, pp. 443-453 (1972)
12. "International Lighting in Controlled Environments Workshop"
T.W.Tibbitts (editor) 1994 NASA-CP-95-3309
http://www.controlledenvironments.org/Light1994Conf/Contents.htm
(see reportes under "photosynthesis")

Big question: can you show me how to rate those effects when building a LED lamp?
Do you go by the lumen scale by any chance?@Pedro
It is from my understanding that is why white is supplemented into an LED array. Mainly cool white. From what I see on the datasheets, they emit plenty along the PAR range and a decent blue burst. The Osram cool whites that is.
Since whites are most efficient on the LED market, it is my opinion and the practiced opinion of some others to use these to increase photysynthetic response during flower. Some state it works well. More white equals bigger buds. What is the rate of diminishing returns? Who knows, thats why I love this new area to play around in. You can now fine tune certain spectra and notice different effects. I have only begun to experiment with LED myself.
Green might have to be added to my array next time for comparison.
That is a great question to ask. Why do you (or anyone else) think researchers limit their research to median wavelengths and not the plethora of assays that could be performed based on this question? Is it just the standard/easiest way? Is that the answer? And, how could you compile the results that would pertain to cannabis as well as other plants; would that even be possible without removing considerable variables? Could you theoretically compile enough data to answer the rate question all in one paper? Seems like that would be a 100+ page paper.
Just thinking out loud. Really enjoyed all the links and discussion.