Utilizing Your JR Peters Lab Results to Select the Proper Jack’s Professional® Fertilizers
Now that you have your water test results, you can begin the process of selecting the correct fertilizer to grow your crops. The first step in the process is to determine the nutritional needs of the plant material you are growing. Once you have this information, you are ready to interpret the results of your water test and begin the process of fertilizer selection.
Step 1: Begin by examining the level of alkalinity in the water.
A simple way to think of alkalinity is as the ability of your water to neutralize acid. The higher the alkalinity, the more acid it will take to lower the pH of your water. Alkalinity is composed of bicarbonates, carbonates and hydroxides joined to calcium, magnesium or sodium. These are the same components found in antacids, such as Tums or Rolaids, baking soda, limestone and lye. Alkalinity is expressed as ppm calcium carbonate equivalent. The higher the number, the more of these components there are in the water. Alkalinity levels are of more concern for crops grown in small containers and for those grown for a long period. Table 1 lists recommended alkalinity ranges for various container sizes.
Table 1. JR Peters Laboratory Alkalinity Guidelines
CONTAINER SIZE
RECOMMENDED RANGE
LEVEL OF CONCERN1
ppm=mg CaCO3/L
Milliequivalents2 CaCO3
ppm=mg CaCO3/L
Milliequivalents CaCO3
Plugs
60-100
1.2-2.0
<40, >120
<0.8, >2.4
Small pots/Shallow flats
80-120
1.6-2.4
<40, >140
<0.8, >2.8
4” to 5” pots/deep flats
100-140
2.0-2.8
<40, >160
<0.8, >3.2
Pots > 6”/long term crops
120-180
1.6-3.6
<60, >200
<1.2, >4.0
1 Alkalinity levels are intended as guidelines only and are dependent on the plant and media type, pot diameter/size, acidity of the feed program and watering practices.
2 Milliequivalents=ppm total alkalinity expressed as mg CaCO3/liter divided by 50.
High alkalinity water may cause a gradual increase in the growing media pH. As the pH climbs, availability of certain plant nutrients, particularly the micronutrients like iron and manganese are negatively affected resulting in deficiencies. It may be necessary to inject mineral acid (sulfuric or phosphoric) into the water or to use acidic media amendments, such as sulfur, or “acid-forming” fertilizers. To determine the amount of mineral acid you need to use to reduce the alkalinity of your water, access the alkalinity calculator located on the web on the North Carolina State University website at http://www.floricultureinfo.com/ and clicking on the floriculture software link. Do not use water that has been water softened. Water softeners add harmful sodium while removing desirable calcium and magnesium. Water softeners do not reduce water alkalinity.
Low alkalinity water usually lacks the components that neutralize acid. As a consequence, the continued use of potentially acidic fertilizers, like many all-purpose formulas, may result in an undesirable decrease in the pH of the growing medium. As the pH drops, certain plant nutrients like iron and manganese may become available in toxic amounts. In addition, these waters are often deficient in calcium, magnesium or sulfate and additional supplements may be needed. A fertilizer program that alternates a potentially basic fertilizer containing calcium and magnesium with a low potential acidity fertilizer can help prevent pH crashes in the growing media and supply needed nutrients.
Step 2: Examine the level of nutrients that may present a concern.
Nutrient levels in water are expected to be low. The exceptions to this rule are calcium, magnesium and sulfur, which frequently occur naturally in water. The normal ranges of plant nutrients in water are listed in Table 2.
Table 2. JR Peters Laboratory Water Quality Guidelines
PARAMETERS
NORMAL RANGE
LOW
HIGH
Soluble Salts (mmhos/cm)
0.3 to 1.0
<0.2
>1.3
M
A
C
R
O
S
Nitrate Nitrogen (NO3-N)
-----
-----
>10
Ammonium Nitrogen (NH4-N)
-----
-----
>10
Phosphorus (P)
-----
-----
>10
Potassium (K)
-----
-----
>10
Calcium (Ca)
40 to 75
<25
>100
Magnesium (Mg)
30 to 50
<15
>50
Sulfur (S)
10 to 80
<10
>80
T
R
A
C
E
S
Manganese (Mn)
-----
-----
>1.50
Iron (Fe)
-----
-----
>2.00
Copper (Cu)
-----
-----
>0.20
Boron (B)*
-----
-----
>0.50
Zinc (Zn)
-----
-----
>0.40
Molybdenum (Mo)
-----
-----
>0.20
O
T
H
E
R
Sodium (Na)
-----
-----
>50
Chlorides (Cl)
-----
-----
>70
Fluorides (F)
-----
-----
>1.0
Aluminum (Al)
-----
-----
>1.0
*Poinsettias are sensitive to boron. A level equal to or greater than 0.25 ppm may be considered high and could cause toxicity.
Nitrogen, phosphorus, and potassium levels greater than 10 ppm may indicate contamination of the water source, possibly due to nutrient runoff; however there is no negative effect on plant growth if these nutrients are present.
High levels of trace elements and other elements, such as sodium and chloride, in irrigation water may result in plant toxicity. Consult with a water treatment specialist to determine the source of these elements and appropriate treatment methods.
Step 3: Determine whether a calcium-containing fertilizer is needed.
Most all-purpose water soluble fertilizers do not contain calcium or magnesium but some water can serve as a source of these essential plant nutrients. If calcium is not supplied by the irrigation water, it is recommended that it be added by a water soluble fertilizer. Most greenhouse crops grow best when they are supplied with a minimum of 50-60 ppm calcium on a continuous basis. Herbaceous perennial and woody plant crops may grow well with slightly lower calcium levels. Poinsettias perform best when continuously supplied with 100-150 ppm calcium. Low calcium levels may be corrected by supplementing with a calcium-containing fertilizer such as Jack’s Professional 15-5-15, 13-2-13, 15-0-15, or 15-0-14.
Sodium is an undesirable component of some waters. Sodium bicarbonate can raise the pH of the growing medium while supplying a potentially toxic element. The best way to minimize the negative effect of sodium in the water is to supply calcium at a level greater than or equal to that of sodium.
Step 4: Check for imbalances and sulfur levels.
The ideal ratio of calcium to magnesium in water or fertilizer solutions is 2 parts calcium to1part magnesium (2:1). Acceptable ratios range from 5:1 to 1:1. Low magnesium levels can be corrected with a drench of Epsom salts (MgSO4 - magnesium sulfate) or by adding Epsom salts to non-calcium containing fertilizers. Sulfur is reported as elemental S. To convert to sulfate (SO4-), multiply by 3. Small amounts of sulfur can be added through the addition of Epsom salts (magnesium sulfate) to non-calcium fertilizer formulations. DO NOT MIX any sulfur-containing compounds with calcium, an insoluble precipitate will form. One ounce of Epsom salts per 100 gallons of water will deliver 7.5 ppm Mg and 30 ppm SO4. The following Jack’s Professional fertilizers contain magnesium in addition to calcium: 15-5-15, 13-2-13, 15-0-14, or 15-4-15. If it is necessary to add more magnesium to a calcium-containing fertilizer, it is possible to add magnesium nitrate, 10-0-0.
Step 5: Consider the nitrogen sources and potential acidity or basicity of the fertilizer.
Nitrogen can be supplied in fertilizers in three forms; nitrate (NO3-), ammonium (NH4+), or urea (CO(NH2)2). The forms of nitrogen in a fertilizer product determine whether the tendency of a particular fertilizer is to raise the pH of the growing medium (potentially basic) or to lower the pH (potentially acidic). The potential acidity or basicity of a fertilizer product is referred to as its CCE (calcium carbonate equivalent) and it is expressed in pounds. This value can be found on the label of the fertilizer. The higher the number, the more effect the fertilizer potentially has to change the pH of the growing medium. The absolute value of this number is not important, but it provides an indication of how strong an acidifier a product may be. High nitrate fertilizers are generally potentially basic while ammoniacal and urea-based fertilizers are generally potentially acidic. Use of a product with high potential acidity may help to prevent the need for mineral acids; however, the pH effect of the fertilizer is not the sole factor to consider when selecting a product.
Products high in the nitrate form of nitrogen tend to produce sturdier, stockier plants, while the ammoniacal and urea forms of nitrogen produce softer, more lush growth. Another factor to consider is that under cool, wet conditions, fertilizers high in ammonium and urea may result in an accumulation of ammonium around plant roots and this may result in ammonium toxicity. For these reasons, many growers select fertilizers with at least 60% nitrate nitrogen for use in the greenhouse, especially during to cool, short days of early spring and when there is an extended period of cool, wet weather. During the high light and warm days of late spring, summer and early autumn, fertilizers with more ammonium and urea may be used.
Step 6: Select fertilizer based on your water quality and crop needs.
After you have evaluated your results for the above factors it is time to select the fertilizer that best fits the needs of the crops you are growing. There is a large selection of fertilizers available that can be mixed and matched to fit your needs. Review the cultural guidelines provided by plant suppliers and modify them as needed based on your geographical and environmental factors. Group plants with similar cultural requirements. For further information of grouping plants, refer to the Jack’s Professional® Technical Bulletin Group Your Plants by Growing Medium pH Preference.
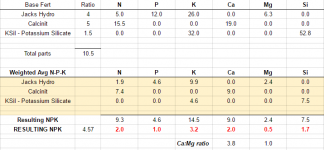
Table 4, below, provides an outline to follow for selecting a proper fertilizer for your water type. Remember, that these are general comments and that your crop may have specific requirements that may alter your selection. Some crop specific characteristics are listed here:
Those tables didn't post correctly, but the point is... Just be sure your starting water quality isn't bad.
Now that you have your water test results, you can begin the process of selecting the correct fertilizer to grow your crops. The first step in the process is to determine the nutritional needs of the plant material you are growing. Once you have this information, you are ready to interpret the results of your water test and begin the process of fertilizer selection.
Step 1: Begin by examining the level of alkalinity in the water.
A simple way to think of alkalinity is as the ability of your water to neutralize acid. The higher the alkalinity, the more acid it will take to lower the pH of your water. Alkalinity is composed of bicarbonates, carbonates and hydroxides joined to calcium, magnesium or sodium. These are the same components found in antacids, such as Tums or Rolaids, baking soda, limestone and lye. Alkalinity is expressed as ppm calcium carbonate equivalent. The higher the number, the more of these components there are in the water. Alkalinity levels are of more concern for crops grown in small containers and for those grown for a long period. Table 1 lists recommended alkalinity ranges for various container sizes.
Table 1. JR Peters Laboratory Alkalinity Guidelines
CONTAINER SIZE
RECOMMENDED RANGE
LEVEL OF CONCERN1
ppm=mg CaCO3/L
Milliequivalents2 CaCO3
ppm=mg CaCO3/L
Milliequivalents CaCO3
Plugs
60-100
1.2-2.0
<40, >120
<0.8, >2.4
Small pots/Shallow flats
80-120
1.6-2.4
<40, >140
<0.8, >2.8
4” to 5” pots/deep flats
100-140
2.0-2.8
<40, >160
<0.8, >3.2
Pots > 6”/long term crops
120-180
1.6-3.6
<60, >200
<1.2, >4.0
1 Alkalinity levels are intended as guidelines only and are dependent on the plant and media type, pot diameter/size, acidity of the feed program and watering practices.
2 Milliequivalents=ppm total alkalinity expressed as mg CaCO3/liter divided by 50.
High alkalinity water may cause a gradual increase in the growing media pH. As the pH climbs, availability of certain plant nutrients, particularly the micronutrients like iron and manganese are negatively affected resulting in deficiencies. It may be necessary to inject mineral acid (sulfuric or phosphoric) into the water or to use acidic media amendments, such as sulfur, or “acid-forming” fertilizers. To determine the amount of mineral acid you need to use to reduce the alkalinity of your water, access the alkalinity calculator located on the web on the North Carolina State University website at http://www.floricultureinfo.com/ and clicking on the floriculture software link. Do not use water that has been water softened. Water softeners add harmful sodium while removing desirable calcium and magnesium. Water softeners do not reduce water alkalinity.
Low alkalinity water usually lacks the components that neutralize acid. As a consequence, the continued use of potentially acidic fertilizers, like many all-purpose formulas, may result in an undesirable decrease in the pH of the growing medium. As the pH drops, certain plant nutrients like iron and manganese may become available in toxic amounts. In addition, these waters are often deficient in calcium, magnesium or sulfate and additional supplements may be needed. A fertilizer program that alternates a potentially basic fertilizer containing calcium and magnesium with a low potential acidity fertilizer can help prevent pH crashes in the growing media and supply needed nutrients.
Step 2: Examine the level of nutrients that may present a concern.
Nutrient levels in water are expected to be low. The exceptions to this rule are calcium, magnesium and sulfur, which frequently occur naturally in water. The normal ranges of plant nutrients in water are listed in Table 2.
Table 2. JR Peters Laboratory Water Quality Guidelines
PARAMETERS
NORMAL RANGE
LOW
HIGH
Soluble Salts (mmhos/cm)
0.3 to 1.0
<0.2
>1.3
M
A
C
R
O
S
Nitrate Nitrogen (NO3-N)
-----
-----
>10
Ammonium Nitrogen (NH4-N)
-----
-----
>10
Phosphorus (P)
-----
-----
>10
Potassium (K)
-----
-----
>10
Calcium (Ca)
40 to 75
<25
>100
Magnesium (Mg)
30 to 50
<15
>50
Sulfur (S)
10 to 80
<10
>80
T
R
A
C
E
S
Manganese (Mn)
-----
-----
>1.50
Iron (Fe)
-----
-----
>2.00
Copper (Cu)
-----
-----
>0.20
Boron (B)*
-----
-----
>0.50
Zinc (Zn)
-----
-----
>0.40
Molybdenum (Mo)
-----
-----
>0.20
O
T
H
E
R
Sodium (Na)
-----
-----
>50
Chlorides (Cl)
-----
-----
>70
Fluorides (F)
-----
-----
>1.0
Aluminum (Al)
-----
-----
>1.0
*Poinsettias are sensitive to boron. A level equal to or greater than 0.25 ppm may be considered high and could cause toxicity.
Nitrogen, phosphorus, and potassium levels greater than 10 ppm may indicate contamination of the water source, possibly due to nutrient runoff; however there is no negative effect on plant growth if these nutrients are present.
High levels of trace elements and other elements, such as sodium and chloride, in irrigation water may result in plant toxicity. Consult with a water treatment specialist to determine the source of these elements and appropriate treatment methods.
Step 3: Determine whether a calcium-containing fertilizer is needed.
Most all-purpose water soluble fertilizers do not contain calcium or magnesium but some water can serve as a source of these essential plant nutrients. If calcium is not supplied by the irrigation water, it is recommended that it be added by a water soluble fertilizer. Most greenhouse crops grow best when they are supplied with a minimum of 50-60 ppm calcium on a continuous basis. Herbaceous perennial and woody plant crops may grow well with slightly lower calcium levels. Poinsettias perform best when continuously supplied with 100-150 ppm calcium. Low calcium levels may be corrected by supplementing with a calcium-containing fertilizer such as Jack’s Professional 15-5-15, 13-2-13, 15-0-15, or 15-0-14.
Sodium is an undesirable component of some waters. Sodium bicarbonate can raise the pH of the growing medium while supplying a potentially toxic element. The best way to minimize the negative effect of sodium in the water is to supply calcium at a level greater than or equal to that of sodium.
Step 4: Check for imbalances and sulfur levels.
The ideal ratio of calcium to magnesium in water or fertilizer solutions is 2 parts calcium to1part magnesium (2:1). Acceptable ratios range from 5:1 to 1:1. Low magnesium levels can be corrected with a drench of Epsom salts (MgSO4 - magnesium sulfate) or by adding Epsom salts to non-calcium containing fertilizers. Sulfur is reported as elemental S. To convert to sulfate (SO4-), multiply by 3. Small amounts of sulfur can be added through the addition of Epsom salts (magnesium sulfate) to non-calcium fertilizer formulations. DO NOT MIX any sulfur-containing compounds with calcium, an insoluble precipitate will form. One ounce of Epsom salts per 100 gallons of water will deliver 7.5 ppm Mg and 30 ppm SO4. The following Jack’s Professional fertilizers contain magnesium in addition to calcium: 15-5-15, 13-2-13, 15-0-14, or 15-4-15. If it is necessary to add more magnesium to a calcium-containing fertilizer, it is possible to add magnesium nitrate, 10-0-0.
Step 5: Consider the nitrogen sources and potential acidity or basicity of the fertilizer.
Nitrogen can be supplied in fertilizers in three forms; nitrate (NO3-), ammonium (NH4+), or urea (CO(NH2)2). The forms of nitrogen in a fertilizer product determine whether the tendency of a particular fertilizer is to raise the pH of the growing medium (potentially basic) or to lower the pH (potentially acidic). The potential acidity or basicity of a fertilizer product is referred to as its CCE (calcium carbonate equivalent) and it is expressed in pounds. This value can be found on the label of the fertilizer. The higher the number, the more effect the fertilizer potentially has to change the pH of the growing medium. The absolute value of this number is not important, but it provides an indication of how strong an acidifier a product may be. High nitrate fertilizers are generally potentially basic while ammoniacal and urea-based fertilizers are generally potentially acidic. Use of a product with high potential acidity may help to prevent the need for mineral acids; however, the pH effect of the fertilizer is not the sole factor to consider when selecting a product.
Products high in the nitrate form of nitrogen tend to produce sturdier, stockier plants, while the ammoniacal and urea forms of nitrogen produce softer, more lush growth. Another factor to consider is that under cool, wet conditions, fertilizers high in ammonium and urea may result in an accumulation of ammonium around plant roots and this may result in ammonium toxicity. For these reasons, many growers select fertilizers with at least 60% nitrate nitrogen for use in the greenhouse, especially during to cool, short days of early spring and when there is an extended period of cool, wet weather. During the high light and warm days of late spring, summer and early autumn, fertilizers with more ammonium and urea may be used.
Step 6: Select fertilizer based on your water quality and crop needs.
After you have evaluated your results for the above factors it is time to select the fertilizer that best fits the needs of the crops you are growing. There is a large selection of fertilizers available that can be mixed and matched to fit your needs. Review the cultural guidelines provided by plant suppliers and modify them as needed based on your geographical and environmental factors. Group plants with similar cultural requirements. For further information of grouping plants, refer to the Jack’s Professional® Technical Bulletin Group Your Plants by Growing Medium pH Preference.
Table 4, below, provides an outline to follow for selecting a proper fertilizer for your water type. Remember, that these are general comments and that your crop may have specific requirements that may alter your selection. Some crop specific characteristics are listed here:
Those tables didn't post correctly, but the point is... Just be sure your starting water quality isn't bad.