acespicoli
Well-known member


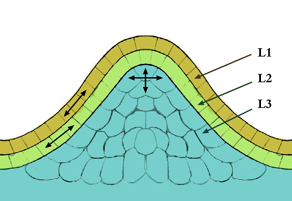
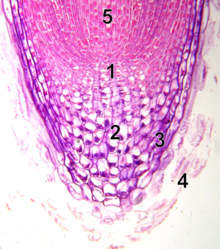
Plant hormone - Wikipedia

Phenotypic plasticity influences the success of clonal propagation in industrial pharmaceutical Cannabis sativa
The burgeoning cannabis market requires evidence-based science such that farmers can quickly and efficiently generate new plants. In part, horticultural operations are limited by the success of cloning procedures. Here, we measured the role of environmental ...

Propagation of Cannabis for Clinical Research: An Approach Towards a Modern Herbal Medicinal Products Development
Cannabis has been reported to contain over 560 different compounds, out of which 120 are cannabinoids. Among the cannabinoids, Δ[9] -tetrahydrocannabinol and cannabidiol are the two major compounds with very different pharmacological profile and ...
Last edited: