Plant hormone - Wikipedia
Phenotypic plasticity influences the success of clonal propagation in industrial pharmaceutical Cannabis sativa - PMC
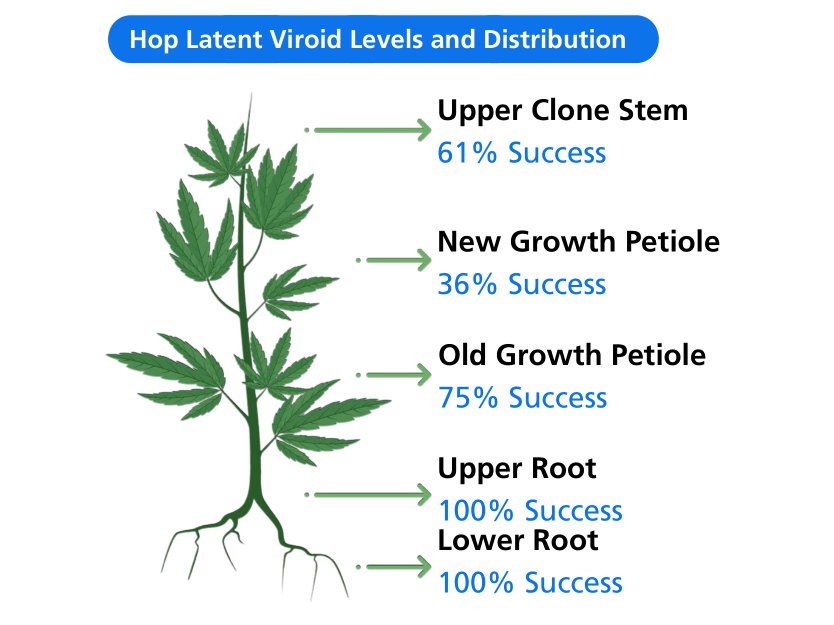
The burgeoning cannabis market requires evidence-based science such that farmers can quickly and efficiently generate new plants. In part, horticultural operations are limited by the success of cloning procedures. Here, we measured the role of ...
Propagation of Cannabis for Clinical Research: An Approach Towards a Modern Herbal Medicinal Products Development - PMC
Cannabis has been reported to contain over 560 different compounds, out of which 120 are cannabinoids. Among the cannabinoids, Δ9-tetrahydrocannabinol and cannabidiol are the two major compounds with very different pharmacological profile and a ...
Clone References
Clone References Work in progress Adams, A. N. (2015). Elimination of viruses from the Hop (Humulus lupulus) by heat therapy and meristem culture. J. Hortic. Sci. 50, 152–160. doi: 10.1080/00221589.1975.11514616 CrossRef Full Text | Google Scholar Braemer, R., and Paris, M. (1987). Biotransfor...
Last edited: