You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Terpenoids
- Thread starter shaggyballs
- Start date
Happy that this one got bumped. I've been interested in the cannabiniod profiles of various clone only medical strains and every
few months I go visit the labs who post their results online to compare the similarity of things like OG, Diesels, GSC, TW, hazes etc.
SC labs in Cali has a 33 point analysis that costs extra but you can find by using the radio buttons
to the left of this website and click on Terpene and then the type of sample you are interested in, flower,hash etc.
http://sclabs.com/tested.html?view=grid&type=Flower,Indica,Sativa&test=Terpene&limit=36&limitstart=0
you will get to an analysis that looks like this:
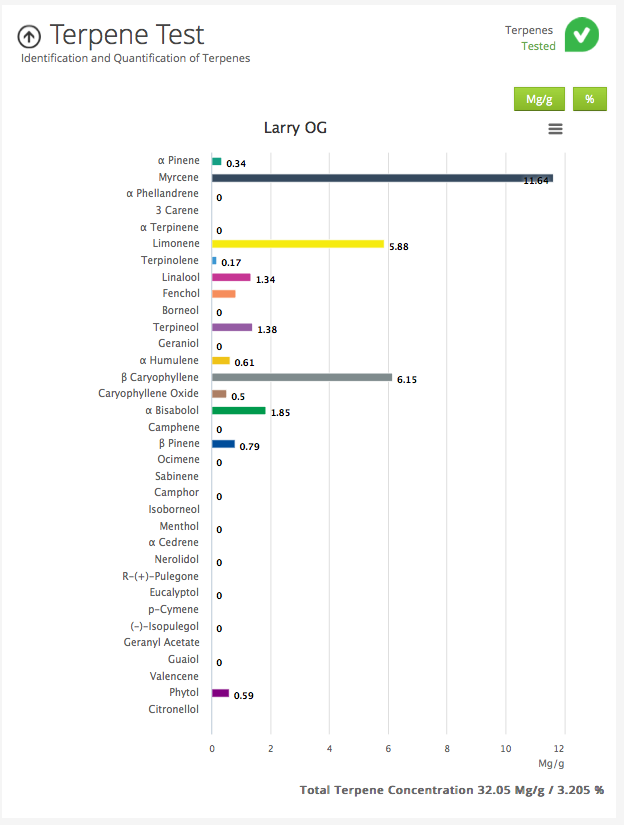
one thing that really interests me is the total percentage of Terpenes by weight that's at the bottom right.
It's like a volume guide to loudness..
I saw one analysis of GG#4 that was 11% Terpenes!
This Seattle lab tests everything for nine Terpenes and gives a percentage weight.
http://analytical360.com/testresults
You can get a good idea of the cannabinoid fingerprints of strains by watching these websites.
few months I go visit the labs who post their results online to compare the similarity of things like OG, Diesels, GSC, TW, hazes etc.
SC labs in Cali has a 33 point analysis that costs extra but you can find by using the radio buttons
to the left of this website and click on Terpene and then the type of sample you are interested in, flower,hash etc.
http://sclabs.com/tested.html?view=grid&type=Flower,Indica,Sativa&test=Terpene&limit=36&limitstart=0
you will get to an analysis that looks like this:
one thing that really interests me is the total percentage of Terpenes by weight that's at the bottom right.
It's like a volume guide to loudness..

I saw one analysis of GG#4 that was 11% Terpenes!
This Seattle lab tests everything for nine Terpenes and gives a percentage weight.
http://analytical360.com/testresults
You can get a good idea of the cannabinoid fingerprints of strains by watching these websites.
Bubbleblower
Member
Has eucalyptol or menthol ever been detected yet?
Can you list what you ordered?
I never saw a Cannabis mix, ever.
Good luck,
-SamS
Hi Sir,
Recentely Restek has manufatured a few standard reference material mix specific for cannabis... like terpene mix...
http://www.restek.com/catalog/view/45361
http://www.restek.com/catalog/view/45362
Also 3 main cannabinoid mix
http://www.restek.com/catalog/view/45362
Peace
what are you working on GrassMan with your analytical equipment?
It must be interesting. I can't believe the price of those Terpenes.
I guess that's probably expected for calibration standards.
Have you come across anything reasonably priced that folks could use to calibrate their nose?
Curious if the Medical Cannabis Terpenes Standard #1 be adequate for that? $220 seems like a good price for 19 Terps.
They are diluting with isopropanol, do you know if it is neutral or affects the smell of the Terps?
Do you think they will sell this to anybody with a credit card or do you need to be a lab?
Great info, thanks for sharing!
It must be interesting. I can't believe the price of those Terpenes.
I guess that's probably expected for calibration standards.
Recentely Restek has manufatured a few standard reference material mix specific for cannabis... like terpene mix...
http://www.restek.com/catalog/view/45361
http://www.restek.com/catalog/view/45362
Have you come across anything reasonably priced that folks could use to calibrate their nose?
Curious if the Medical Cannabis Terpenes Standard #1 be adequate for that? $220 seems like a good price for 19 Terps.
They are diluting with isopropanol, do you know if it is neutral or affects the smell of the Terps?
Do you think they will sell this to anybody with a credit card or do you need to be a lab?
Great info, thanks for sharing!
after looking at their website closer it appears the 19 are mixed together into 2 vials.
"Prepared in two separate multicomponent mixes to maximize stability."
bummer.
"Prepared in two separate multicomponent mixes to maximize stability."
bummer.
Hi,
I'm just trying to understand better ratios and synergies... at least that's what I'm able to tell...
Those prices are for standard reference material to calibrate the GC. That's why they are expensive.
The Restek mix is diluted and a mix of different terpenoids on the same concentration, so it won't be useful to educate the nose.
The ones that I posted before are not diluted, almost pure form (more than 99% purity) and can be used for educate nose but the price is still expensive.
I've been pointed to this web to purchase terpenoids for educating nose and extract formulations but didn't check them yet.
http://extractconsultants.com/product-category/terpenes-and-aroma/
No idea regarding the option to purchase them without been a lab.
Theorically you could purchase them if you are a bussiness. Those are not controlled substances.
Not exactely. The first mix contains 19 terpenoids.
(-)-alpha-Bisabolol (23089-26-1)
Camphene (79-92-5)
delta-3-Carene (13466-78-9)
beta-Caryophyllene (87-44-5)
Geraniol (106-24-1)
(-)-Guaiol (489-86-1)
alpha-Humulene (6753-98-6)
p-Isopropyltoluene (p-cymene) (99-87-6)
(-)-Isopulegol (89-79-2)
d-Limonene (5989-27-5)
Linalool (78-70-6)
beta-Myrcene (123-35-3)
Nerolidol (7212-44-4)
Ocimene (13877-91-3)
alpha-Pinene (80-56-8)
(-)-beta-Pinene (18172-67-3)
alpha-Terpinene (99-86-5)
gamma-Terpinene (99-85-4)
Terpinolene (586-62-9)
And the second mix contains 2 terpenoids.
(-)-Caryophyllene oxide (1139-30-6)
1,8-Cineole (Eucalyptol) (470-82-6)
On analytical chemistry is very useful to get from a certified supplier a mix of many compounds that you want to quantify.
Think that, in order to do proper work, each sequence of analysis start by a calibration curve of different points (I like at least 5 points) which is prepared mixing all the compounds in 5 different vials at different concentrations.
That means that if I want to quantify 30 terpenoids and the supplier sells them independentely, I need prepare 5 vials where I need to mix 30 terpenoids on each one at different volumes. One mistake in one terpenoid means to start again the mix.
You are welcomed. And thanks to you for the info you spread aswell.
Peace.
what are you working on GrassMan with your analytical equipment?
It must be interesting. I can't believe the price of those Terpenes.
I guess that's probably expected for calibration standards.
Have you come across anything reasonably priced that folks could use to calibrate their nose?
Curious if the Medical Cannabis Terpenes Standard #1 be adequate for that? $220 seems like a good price for 19 Terps.
They are diluting with isopropanol, do you know if it is neutral or affects the smell of the Terps?
Do you think they will sell this to anybody with a credit card or do you need to be a lab?
Great info, thanks for sharing!
I'm just trying to understand better ratios and synergies... at least that's what I'm able to tell...
Those prices are for standard reference material to calibrate the GC. That's why they are expensive.
The Restek mix is diluted and a mix of different terpenoids on the same concentration, so it won't be useful to educate the nose.
The ones that I posted before are not diluted, almost pure form (more than 99% purity) and can be used for educate nose but the price is still expensive.
I've been pointed to this web to purchase terpenoids for educating nose and extract formulations but didn't check them yet.
http://extractconsultants.com/product-category/terpenes-and-aroma/
No idea regarding the option to purchase them without been a lab.
Theorically you could purchase them if you are a bussiness. Those are not controlled substances.
after looking at their website closer it appears the 19 are mixed together into 2 vials.
"Prepared in two separate multicomponent mixes to maximize stability."
bummer.
Not exactely. The first mix contains 19 terpenoids.
(-)-alpha-Bisabolol (23089-26-1)
Camphene (79-92-5)
delta-3-Carene (13466-78-9)
beta-Caryophyllene (87-44-5)
Geraniol (106-24-1)
(-)-Guaiol (489-86-1)
alpha-Humulene (6753-98-6)
p-Isopropyltoluene (p-cymene) (99-87-6)
(-)-Isopulegol (89-79-2)
d-Limonene (5989-27-5)
Linalool (78-70-6)
beta-Myrcene (123-35-3)
Nerolidol (7212-44-4)
Ocimene (13877-91-3)
alpha-Pinene (80-56-8)
(-)-beta-Pinene (18172-67-3)
alpha-Terpinene (99-86-5)
gamma-Terpinene (99-85-4)
Terpinolene (586-62-9)
And the second mix contains 2 terpenoids.
(-)-Caryophyllene oxide (1139-30-6)
1,8-Cineole (Eucalyptol) (470-82-6)
On analytical chemistry is very useful to get from a certified supplier a mix of many compounds that you want to quantify.
Think that, in order to do proper work, each sequence of analysis start by a calibration curve of different points (I like at least 5 points) which is prepared mixing all the compounds in 5 different vials at different concentrations.
That means that if I want to quantify 30 terpenoids and the supplier sells them independentely, I need prepare 5 vials where I need to mix 30 terpenoids on each one at different volumes. One mistake in one terpenoid means to start again the mix.
@Grassman
Thanks deeply for the Restek resource......
You are welcomed. And thanks to you for the info you spread aswell.
Peace.
Not exactely. The first mix contains 19 terpenoids.
(-)-alpha-Bisabolol (23089-26-1)
Camphene (79-92-5)
delta-3-Carene (13466-78-9)
beta-Caryophyllene (87-44-5)
Geraniol (106-24-1)
(-)-Guaiol (489-86-1)
alpha-Humulene (6753-98-6)
p-Isopropyltoluene (p-cymene) (99-87-6)
(-)-Isopulegol (89-79-2)
d-Limonene (5989-27-5)
Linalool (78-70-6)
beta-Myrcene (123-35-3)
Nerolidol (7212-44-4)
Ocimene (13877-91-3)
alpha-Pinene (80-56-8)
(-)-beta-Pinene (18172-67-3)
alpha-Terpinene (99-86-5)
gamma-Terpinene (99-85-4)
Terpinolene (586-62-9)
Hey bro what's up? That's great, a premade cannabis terpene selection! Although I think a smaller or different selection from the most dominant ones could be more useful (and enough) for non R&D purspuses and educating the nose. From all the papers I've been reading, the most prevailing terpenes almost always:
Monoterpenes:
b-myrcene (hops, lemongrass)
r-limonene (lime, oranges and other citrics) and s-limonene (pine, turpentine)
s-linalool (coriander) and r-linalool (lavender, basil)
ocimene (lime)
terpinene (cardamom oil)
terpinolene (pine oil)
Sesquiterpenes:
a-Pinene (pine and conifer oil)
b-Pinene (pine and conifer oil)
a-humulene (hops)
b-caryophyllene (trans- and oxide forms too, commonly found in pepper and clover)
The rest are present in such small amounts that it's probably hard to perceive and isolate them by the nose. I'd like to experiment as well with Cadinene (juniperus oil), Cineol (eucalyptus oil), Citral (lemon myrtle, lemongrass), Borneol (camphor) and Menthol (mint oil) but they are found mostly as traces in certain strains. The selection I made are almost present within all strains at bigger or smaller amounts:

Chart featuring terpenes found in 19 different fiber and drug cultivars:

Shame that Extract Consultants won't ship overseas because they have a great selection at reasonable prices for those without access to analytical standards but still wanting to experiment with this compounds.
I've researched and found as well that monoterpenes levels drop drastically after drying the flowers for a few days. While the sesquiterpenes tend to stay and even most rise with the curing. That fact is definitely the reason why fresh buds smell more pungent and fruity/sweet than the warmer woody and spicy well cured buds. Something that many of us have learned empirically.
That together with the degradation and oxidation of THC>CBN is definitely the reason why the fresher weed has a sharper effect and higher potency than the softer smelling, warmer and more relaxing 1+ year cured herb.
Has eucalyptol or menthol ever been detected yet?
Eucalyptol (1,8-Cineole) has certainly been detected. See my previous post.
Happy that this one got bumped. I've been interested in the cannabiniod profiles of various clone only medical strains and every few months I go visit the labs who post their results online to compare the similarity of things like OG, Diesels, GSC, TW, hazes etc.
Has it been your impression that there really is enough similarity, to make a distinctive fingerprint? And various labs all fairly consistently get the same profile?
x
Last edited:
Here is a good video long but on topic.
Here is a good video long but on topic.
-
Here is a good video long but on topic.
-
superglue
Member
Marijuana comes in many different flavors
What Are Terpenes? vid
Terpene Interplay vid
A terpene analysis is like a fingerprint
Terpene:
1). Naturally occurring hydrocarbons, emitted by many trees and plants. They mostly have very strong smells and are responsible for the aromas of the vegetation in which they are found. Terpenes can be thought of as being built from units of isoprene, C 5 H 8 , joined together into chains and rings. Monoterpenes, formula C 10 H 16 , constitute the major emissions from conifers and fruit trees. Sesquiterpenes, formula C 15 H 24 , are commonly found in citrus trees.
2). Monoterpenes and triterpenes comprise the terpenes under investigation. Most of the attention is focused on two monoterpenes: limonene and perillyl alcohol.
Each strain has a unique genetic potential. The grower's expertise determines how close to the plant comes to reaching that potential. Every harvest is often slightly different due to subtle changes in the environment
Terpenes are volatile compounds produced by many plants, as well as some insects. Plants that produce terpenes often possess smells and flavors we find pleasing and are known as aromatic herbs. These aromatic plants have been used by cultures around the world, not only for perfumery and cooking, but also as medicine. The distinctive flavor and smell of each aromatic plant is caused by its unique blend of terpenes. 120 distinct terpenes are produced by the genus Cannabis, with the relative concentrations of the individual terpenes varying greatly among the 700 distinct strains currently in cultivation.
Aside from taste and smell differences between varieties, this helps contribute to the broad diversity of potential medical applications of Cannabis. Laboratory experiments have shown that the full range of psychoactive and medical effects of Cannabis resin cannot be re-created simply with the use of pure cannabinoid type drugs like THC (tetrahydrocannabinol). Marinol and Dronabinol, two drugs containing synthetic THC that have demonstrated limited medical benefits when compared with the use of Cannabis material containing the full range of cannabinoids and terpenes. These observations indicate that in addition to the psychoactive properties present in Cannabis resin, secondary components including terpenes are either psychoactive themselves, or are able to modulate or potentiate the affect of the cannabinoids when ingested in combination. GW Pharmaceuticals has invested extensive research into Cannabis based medicines, concluding that terpenes played a significant role in the effectiveness of the medication. GW is now manufacturing the most widely used medical marijuana product in the world an oral spray called Sativex, which contains a standardized mixture of Cannabis terpenes in addition to a mix of THC and CBD (Canabidiol).
From a chemical standpoint, terpenes are a large and varied class of hydrocarbons that make up a majority of plant resins and saps. The name “terpene” comes from turpentine, a terpene-based solvent distilled from pinesap. Essential oils, composed primarily of terpenes, have a long history of topical and internal medicinal use. Cannabinoids like THC are chemically classified as terpenoids, meaning they are derived from terpenes themselves. This explains the common practice among marijuana users of judging the quality of dried cannabis or hashish based largely on the quality and intensity of the smell. In high-THC cultivars, because the THC is made from terpenes, their content is usually correlated with psycho activity.
The resinous trichromes of the cannabis plant contain both the cannabinoids as well as the terpenes, which are constantly being replaced as they evaporate from the resin. The resin of high THC cannabis contains approximately 20 percent terpenes, and 50 percent cannabinoids by weight. The essential oil has traditionally been used as a treatment for skin conditions such as eczema or psoriasis, as a topical antibiotic agent, and to increase circulation. In addition to these topical uses, it is now known that terpenes present in Cannabis do possess neurological effects, altering the production of the neurotransmitters seratonin and dopamine, as well as acting as type 2 cannabinoid receptor agonists. Another significant action when used in combination with cannabinoids is their ability to alter the permeability of both cell membranes and the blood/brain barrier, causing THC and other active cannabinoids to have a faster onset and more thorough absorption. Myrcene and several other terpenes are known to act as mixed agonist/antagonists of cannabinoid receptors, modulating the effects of THC in a similar fashion to CBD (cannabidiol).
The Major Terpenes of Cannabis Resin and Their Effects
Borneol – Borneol is a major component of cannabis resin that can also be found in cinnamon and wormwood (Artemesia spp). In Chinese medicine herbs containing borneol are recommended for fatigue and overstress. Borneal is mentioned to be a calming sedative.
Corryphyllene – Corryphyllene is a major component of cannabis resin that can also be found in black pepper and cloves. It is a fairly weak agonist of the type 2 cannabinoid receptors (cb2). As a constituent of a salve or lotion corphyllene is an effective anti- inflammatory and analgesic. Drug dogs are trained to specifically sniff out corphyllene epoxide, a similar compound produced only by cannabis.
Cineole/eucalyptol – Cineole/eucalyptol content is quite variable across varieties of Cannabis, but is often a major component of the essential oil. It is also found in rosemary and eucalyptus and is used to increase circulation, and reduce pain and swelling when applied topically. It readily crosses the blood/brain barrier, possibly helping cannabinoids to cross more readily as well. The effects of cineole, when combined with oral or smoked Cannabis, are reported as being very uplifting, noticeably increasing mental and physical energy. This terpene, or others like it, may be responsible for the reported difference in effect between indica and sativa strains with a similar cannabinoid profile.
Limonene – Found in cannabis resin as well as tropical fruit rinds, limonene is an anti-bacterial, anti fungal and anti cancer agent. Currently undergoing trials for use as an anti depressant, it is also known to increase the absorption of other terpenes by making cell membranes more permeable. The presence of this anti fungal agent may be helpful in protecting against Aspergillus infection in those with compromised immunity when using spoiled or poorly cured marijuana. Limonene is currently in trials to study its ability to prevent breast cancer formation.
Delta-3-Carene – A component of cannabis, rosemary, pine, and cedar resin. Aromatherapy oils that contain high levels of delta3carene are used to dry excess fluids from the eyes, nose, or mouth. It is thought to be at least partially responsible for the dry mouth and eye problems that are common side effects of the use of cannabis.
Linalool – This major component of cannabis and lavender oils is believed to possess anti anxiety and sedative properties. Strains that are high in linalool and similar compounds may be particularly beneficial for patients who experience insomnia when consuming Cannabis.
Myrcene – Significant concentrations of myrcene are present in cannabis resin. It is also found in mango, hops, lemon grass, East Indian bay tree, and verbena. Because of its appealing fragrance, myrcene is used in the perfume industry. It has a similar modulating effect on the binding of Cannabinoid agonist drugs as Cannabidiol, possibly reducing effects of Cannabis resin that are found to be unpleasant for some medical users. It has anti microbial, anti septic, analgesic, anti oxidant, anti carcinogen and anti-inflammatory properties. It has shown some promise when used as an anti depressant, or as an additive to other anti depressant drugs and is also used in massage therapy as a muscle relaxer.
Terpineol – Minor component of Cannabis resin, used extensively in the perfume industry. Interestingly this terpene decreases motility of lab rats by 45 percent, this observation coupled with the fact that this is a terpene produced primarily in Cannabis indica plants indicates terpineol could play a role in decreased motility sometimes referred to as “couch lock”.
View Image
To get the greatest possible benefits from medical Cannabis products, its important to be aware of the common methods being used to produce this medication, and how this will affect the terpene content of the finished product. When Cannabis is exposed to heat the volatile terpenes quickly evaporate, causing the majority of hash oils currently produced for medication to be nearly devoid of terpenes. When purchasing hash oil products it is important to ask if the terpenes have been retained during processing. Ask your dispensary staff if they are aware of the manufacturing processes used in their products, and the properties of the finished medicines.
Cannabis-based salves or lotions have become a popular treatment for skin conditions, and terpenes play a major role in the effectiveness of these at treating a range of skin problems. When purchasing these types of products you should talk to your dispensary about the terpene content of the different products available as well as the cannabinoid content. When topically applied, cannabis terpenes are very effective for treating a range of skin problems. If you are a medical marijuana user who prefers to smoke or vaporize cannabis, you can increase the effect of the terpenes in you’re bud by slowly breaking it up and inhaling the aromas prior to smoking. Some concentrates that have become popular retain very little of the original terpenes. This is true of most hash oils that are extracted or dried with heat, as well as bubble hash that have lost much of the original terpenes to the water used in processing. The most concentrated terpenes are found in freshly dried buds, as well as high quality dry sift hash or kief.
It is now understood that the psychoactive and medicinal effects of the cannabis plant can’t be explained by THC and other cannabinoids alone. In order to develop a more thorough understanding of the range of medical conditions alleviated with Cannabis use, terpenes, flavonoids and alkaloids that are produced by different strains of cannabis will need to be studied to determine how they interact with cannabinoids to produce the unique healing properties of organic Cannabis resin.
The importance of terpenes in medicinal cannabis is becoming more evident as the research progresses. They are responsible for many of the subtle differences between strains and in how they perform medically.
As patients become more aware of the complexities of the various compounds in cannabis, they will become more discerning when choosing their medicines. Hopefully, as patients become more aware of the full potential of Cannabis preparations, it will help increase the quality of available medications, and the quality of information. Educated patients can be the driving force for further research into the almost limitless potential for the medical uses of this amazing plant.
Terpenes May Improve Effectiveness Of Medical Marijuana
Terpenes Influence The Synergy Effect Of Cannabis
As we know, science has identified and characterized the molecular structure of around 20,000 terpenes, which makes it the largest category of plant chemicals. These aromatic compounds are found in the essential oils of plants and flowers, and plenty of studies have been done on their effects.
Of the 20,000 identified terpenes, there have been more than 120 found in cannabis. Only a few of them appear in high concentrations, but they have been found to have a number of benefits. A few of these effects are covered in our terpenoid article, but recent research has suggested an “entourage effect” as well. In his 2011 study “Taming THC,” Ethan Russo, from GW Pharmaceuticals, discussed the interaction between terpenes and cannabinoids.
Terpenes May Reduce THC-Induced Anxiety
“Citrus fruits (high in limonene) were used as a ‘cannabis antidote’ in 10th century Persia.”
For years, tetrahydrocannabinol (THC) was the only cannabinoid investigated for its medicinal value, and we know it has the potential to cause anxiety in some patients. However, certain terpenes in cannabis, like Linalool, have been found to counter the anxiety.
In fact, Russo points out that terpenes likely played a role in a number of ancient antidotes for the less desirable effects of THC. For instance, citrus fruits (high in limonene) were used as a “cannabis antidote” in 10th century Persia. Other ancient antidotes include calamus plant roots and pine nuts (high in pinene), as well as black pepper (high in caryophyllene and myrcene).
Terpenes And Cannabinoids Can Work Together
Terpenoids can be used for more than countering THC-induced anxiety. Russo discussed interactions to treat a number of issues including: pain, inflammation, depression, addiction, epilepsy, cancer, and infections.
Russo believes pinene would be useful in the treatment of MRSA. Cannabigerol (CBG) is a potent MRSA inhibitor, and can be found with small amounts of THC. Because of this Russo suggests a whole-plant extract, high in CBG and pinene, which was found to have its own anti-MRSA qualities in 2010.
“Combining terpenoids with a CBD-rich extract may help treat the wide-ranging effects of Alzheimer’s disease.”
Terpenes could also aid in Alzheimer’s treatment with cannabidiol (CBD). Linalool, which is prominent in lavender, helps counter stress and anxiety. Limonene is commonly used in aromatherapy to improve mood, and pinene is known to promote alertness and memory retention. Combining these terpenoids with a CBD-rich extract may help treat the wide-ranging effects of Alzheimer’s disease.
Another interaction that Russo highlighted could have benefits for addiction treatment. An essential oil made from black pepper reduced nicotine cravings in cigarette smokers. Interestingly enough, black pepper essential oils are high in myrcene, pinene, and caryophyllene, all of which can be found in cannabis.
Caryophyllene is interesting because it directly stimulates the CB2 receptors throughout the body. As we know, CB2 agonists prevent the release of dopamine, which is related to addiction. This, in combination with the use of CBD for opiate withdrawal, suggests that cannabis with caryophyllene could have a variety of rehabilitative benefits.
“K”, like multiple growers on online forums, believes that a number of variables (lighting, soil composition, nutrients, etc.) can influence terpene production. If growers are able to influence the production of specific terpenes, they could improve their product’s effectiveness.
Terpenes Can Improve Medical Marijuana, Infused Products
It’s been reported that certain terpenes dilate capillaries in the lungs. Logic tells us that this would be useful in the case of smoked or vaporized cannabis. Dilated capillaries would enable beneficial cannabinoids to enter the bloodstream easier. This certainly could be useful for growers who know how their crops will be ingested, and in the production of cannabis concentrates.
In fact, a number of concentrate makers enhance their finished product with pure terpenes. This is typically done for added flavor, as the more volatile terpenes can be lost during the extraction process. However, infusing concentrates with a specific terpene for added effect would be equally beneficial. For instance, pinene is a bronchodilator, which could benefit asthma patients.
In fact, similar processes already exist. According to Jeff Raber, founder of The Werc Shop, a lab-testing facility in Los Angeles, they are able to infuse concentrates with the terpenes lost. “Based on the terpene-profile of each strain,” he added, “we can recreate as much of the whole plant component as possible.”
One step further, K believes terpene-rich extracts could play a major role in the future of medical marijuana. He points out that some patients might want the terpene-related flavor and relief, without the high from THC.
Another potential application of terpenes could benefit users of medicated topicals. Nerolidol, a sedative terpene, is a known skin penetrant. Therefore, it could aid in cannabinoid absorption if infused in topicals.
The benefits of terpenes are widely recognized, but they just now are being explored by experts in the cannabis industry. As Ethan Russo pointed out, terpenes may influence a number of cannabis’ benefits. Their interaction with cannabinoids often impacts the effectiveness of medical marijuana strains and products, and could be used to facilitate a better overall experience.
I found a paper (Ross, S.A.; ElSohly, M.A. J. Nat. Prod. 1996, 59: 49-51) that notes the % of essential oils (terpenes) in fresh cannabis flowers to be .29 %. The major components of that essential oil fraction are myrcene (67%) and limonene (16%). That works out to 1.9 mg of myrcene and .46 mg of limonene per 1.0 g of fresh cannabis flowers.
http://berkeleypatientscare.com/2010/10/08/terpenes-terpenoids-and-cannabis/
Organoleptic tests will reveal little about the %'s or terpene contents of the over 130 terpenes, and total yields are so affected by the environment and harvest dates that it is hard to eliminate variables.
You need analytical testing to be honest. Breeding requires it.
I am not so sure what the nose can tell, on ID'ing the 130+ terpenes. But I do agree that I can often pick out a best plant from seeds of one variety, just from smells, assuming that they are similar in yields, flower to leaf ratio, and other important factors.
And once you understand what each terpene or combination of terpenes contributes then analytical results will allow predictions without smelling or smoking, it is not so far away.
Breeding terpenes is certainly possible.
-SamS
Terpene biosynthesis, as with the biosynthesis or production of other small molecules, is controlled by genes that encode the involved enzymes. However, regulation of these genes and enzymes takes place and can greatly affect terpene production. I know for a fact that the enzymes involved in terpene biosynthesis (not shown in MJ to the best of my knowledge but other models) are affected by the availability of their respective metal cofactors, such as cobalt and magnesium. But often times excess cofactors lead to inhibition, so dumping in extra epsom salt is not a good idea. Other factors such as temperature and humidity are likely regulators of terpene biosynthesis, but only time, money, and research will tell. It would be exciting to uncover the exact conditions that promote maximum terpene synthesis.
https://www.icmag.com/ic/showthread.php?t=245790&highlight=Terpenes
Growth regulators and essential oil production
This review will focus on the effects of exogenous application of growth regulators on essential oil production of various species and the factors responsible for them.
Essential oils: Among the diversity of secondary metabolite classes we found the isoprenoids (also terpenes or terpenoids), whose name is related to its five-carbon structure: isopentenyl diphosphate (IPP). Isoprenoids occur in plants as primary metabolites (ubiquinone, plastoquinones, gibberellins, brassinosteroids, carotenoids and others) (Rodriguez-Concepción and Boronato 2002). However, isoprenoids classified as secondary metabolites are very important due to their ecological functions like attraction of pollinators and seed dispersion, protection against herbivores and allelophaty (Paré and Tumlinson 1999; Wink 2003).
Terpenes (the usual name) are biosynthesized through two pathways: mevalonate and methylerythritol phosphate (Figure 1). The first is located in the cytosol and endoplasmatic reticulum (Hadacek 2002), which has acetyl-CoA as its precursor, while the second occurs in the plastids from glyceraldehyde-3-phosphate and pyruvate (Rodriguez-Concepción and Boronato 2002). Both generate isopentenyl diphosphate (IPP), which is isomerized (isopentenyl diphosphate isomerase) forming dimethylallyl diphosphate (DMAPP), the isoprene synthase substrate, an enzyme that is present in the chloroplast responsible for the diphosphate break and isoprene (a five-carbon compound) formation. Adding an IPP molecule to DMAPP through prenyltransferases will generate geranyl diphosphate (GPP), a monoterpene (C10) precursor. Consecutive condensation of IPP (by special prenyltransferases) produces farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGDP), which are precursors of sesquiterpenes (C15) and diterpenes (C20), respectively (Figure 2). These terpene groups will be converted by terpene synthases giving rise to other compounds. There are also triterpenes, tetraterpenes and politerpenes, with 30, 40 and more than 45 carbons, respectively (Bohlmann et al. 1998; Trapp and Croteau 2001; Bohlmann and Keeling 2008).
Terpene synthases products may suffer many reactions (oxidation, reduction, isomerization, conjugation, etc) giving rise to stereochemicals and metabolic variants (Kesselmeier and Staudt 1999; Sangwan et al. 2001), which deliver a range of chemical diversity found in this secondary metabolite class.
The production and accumulation of essencial oils are related to specialized structures since they are very toxic to cells. There are numerous sorts of specialized secretory structures like, for instance, glandular thricomes, secretory cavities, idioblasts and others (Gershenzon 1994). According to Gottlieb and Salatino (1987) the essential oil production and the secretory structure formation are closely connected.
Endogenous factors like development stage of whole plant and specific organs, and exogenous factors (biotic and abiotic) can alter essential oil production (Sangwan et al. 2001; Lima et al. 2003; Gobbo-Neto and Lopes 2007).
In a comprehensive review about essential oil production regulation Sangwan et al. (2001) indicated ontogeny, photosynthetic rate, photoperiod, light quality, climatic and seasonal changes, nutrition, humidity, salinity, temperature, storage structures and growth regulators as factors that alter quantitatively and qualitatively the production of this class of compounds.
According to Farooqi and Shukla (1999) growth regulators, or plant hormones, stimulate plant growth and terpene biosynthesis in a broad number of aromatic plant species, which result in beneficial changes in terpene quality and quantity.
The use of growth regulators in agricultural production has increased due to their positive influence on product quality. This is a common practice in small countries where this technology is necessary to achieve higher yields and better products (Poyo and Ono 2006).
It is known that plant growth and development are regulated by action and balance of different groups of growth regulators, which promote or inhibit such processes. Nevertheless, the effects of the use of plant growth regulators on essential oil production are not well known (Ortuño et al. 1999; Poyo and Ono 2006).
Plant Growth Regulators - Phytohormones: Plant growth regulator is a term which includes hormonal substances of natural occurrence (phytohormones) as well their synthetic analogues (Basra 2000).
The concept of phytohormone was proposed by Julian von Sachs at the end of 19th Century, who characterized them as mobile endogenous compounds acting as organ formers (Spartz and Gray 2008). Phytohormones are simple molecules that have specific effects on plant growth and are active at low concentrations (Nambara and Marion-Poll 2005; Teale et al. 2006).
All the aspects of plant growth and development are under phytohormone control. A single phytohormone can regulate a wide range of processes. On the other hand, a unique process can be regulated by the action of many plant hormones. Although nowadays the use of mutants is a valuable tool to clarify hormone functions, traditionally the physiological effects of diverse plant hormones has been established by their exogenous application (Gray 2004).
Five classes of phytohormones are classic:
auxins (1), cytokinins (2), gibberellins (3), abscisic acid (4) and ethylene (Nambara e Marion-Poll, 2005). In addition, compounds like jasmonate (5) and brassinosteroids (6) are also classified as plant hormones (Taiz and Zeiger 2004).
Auxins (AUX) act from the embryo formation until tropic stimulus processes, but are known as growth hormone due to their role in cell elongation. The response to auxin includes regulation of a broad number of genes. In plants the predominant auxin is indol acetic acid (IAA). There is a large biosynthesis of auxin in young tissues on the shoot as well in the root meristematic apices. Auxin is distinguished from the other hormones by its specific and active transport, which is one of the factors that influence the activity of this hormone (Pozo et al. 2005; Teale et al. 2006; Spartz and Gray 2008).
Cytokinin (CYT) is a phytohormone that participates in events in the course of whole plant ontogeny, from fecundated ovule to senescence and death. It is present in processes such as cell division, shoot initiation and growth, senescence delay and photomorfogenic development, control of chloroplast division and growth, modulation of metabolism and morphogenesis in response to environmental stimulus (Chenyad'ev 2000; Kieber 2002; Pozo et al. 2005; Hirose et al. 2007).
Gibberellins (GA) are regulators of plant height. They are diterpenes which regulate stem elongation, seed germination and flowering. They are also associated with juvenile to adult transition processes, promote fructification and play an important role on seed germination by activation of embryo vegetative growth and mobilization of energetic reserves from endosperm (Taiz and Zeiger 2004; Spartz and Gray 2008).
Abscisic acid (ABA) has great importance in developmental processes and seed germination, as the induction of seed dormancy, protein and lipid synthesis, tolerance to desiccation and inhibition of the embryonic to vegetative development. In mature plants ABA acts on the response to drought through stomata aperture. It also acts on adaptation to stress conditions like low temperature, salinity, hypoxia and in response to pathogen attacks. In a general way ABA is considered a hormone with inhibitory activity on growth (Nambara and Marion-Poll 2005; Pozo et al. 2005).
Ethylene (ET) is a phytohormone that acts on seed germination, shoot and root growth, flower development, flower and leaf senescence and abscission and fruit maturation. Its particularity is the emission by diffusion as a gas. It is also associated with plant defense, acting on the induction of xylem inclusions and phytoalexins synthesis. Ethylene production can be induced by factors like drought, inundation, ozone exposure, or mechanical injury, which associate it with stress responses (Taiz and Zeiger 2004; Adie et al. 2007).
Among the other phytohormones, jasmonate (JA) is distinguished by its association with processes of response to herbivore and pathogen attacks through chemical defense inductions like phytoalexin biosynthesis. Furthermore, jasmonate regulates a variety of responses to abiotic stress, as well as processes associated with reproduction and senescence. The physiological effects are not restricted to jasmonic acid, but to a broad range of compounds including precursors and conjugated which acts as signaling molecules on plant development and adaptation to stress conditions (Schaller and others 2005; Wasternack 2007; Spartz and Gray 2008).
Brassinosteroid (BR) is a phytohormone that acts regulating cell elongation and division. It affects plant curvature, reproductive and vascular development, membrane polarization and proton pumping, the source-sink relationship, and stress modulation through interaction with environmental signs. Brassinosteroid levels vary among tissues, but the mainly sources are grains of pollen and immature seeds. Normally immature tissues have a higher brassinosteroid concentration, which explains its effect on young tissues. Mutant plants to brassinosteroids are dwarf and have a growth pattern similar to plants exposed to light even when it is absent. These mutants show altered leaf morphology when exposed to light (Clousse and Sasse 1998).
Essential oil production using plant growth regulators:
The effects of growth regulators (Figure 4) on essential oil production, by means of leaf spraying or in vitro culture systems, are very variable. Changes only on yield or content of essential oil could be verified. In some cases chemical changes also occur, in others no effect is noticed. If taken into account that growth regulators influence plant growth and development, affecting physiological and biochemical processes, or even gene regulation, there are a great number of ways in which applications of those compounds could alter the essential oil production (Shukla and Farooqi 1990).
One of the manners of influencing essential oil production is through effects upon plant growth. The induction of leaf and flower production or a general increase in biomass can result in higher essential oil yield. In mint (Mentha arvensis) the use of 200 ppm of cinetine (7) resulted in an increase of biomass production which, according to Farooqi and others (2003), contributed to a rise in essential oil yield. In another assay with M. arvensis L. var. piperascens Mal., Farooqi and Sharma (1988) verified that a decrease in plant size associated with a higher leaf production was the cause of the increment in essential oil yield in plants treated with cytokinins and naphthalene acetic acid (NAA) (8).
Essential oils occur throughout the plant, but they are frequently found in flowers and leaves. Poyh and Ono (2006) observed in sage (Salvia officinalis) treated with 100 mg L-1 of gibberellic acid (GA) higher essential oil content compared to control plants. According to the authors, this was a result of an increase in leaf number. Application of ethrel [(2-chloroethyl) phosphonic acid] (9), which is degraded when in contact with plant cells producing ethylene, in concentrations of 50 and 100 mg L-1, resulted in a decrease in plant height, yet there was an increase of 38-42% on fresh and dry mass of flowers in relation to control plants. On the other hand, high concentrations (250 and 500 mg L-1) had negative effects not only on plant height, but also on flower production. Nevertheless, single flower mass was not influenced (Haque and others 2007). Applications of different forms of brassinosteroid analogues (ketonic and lactonic spirostane) resulted in an increase of fresh matter of leaves and higher menthol production in Mentha arvensis L. (Maia et al. 2004).
Due to its high toxicity essential oils are biosynthesized and stored in specialized structures (Gershenzon 1994). Thus the occurrence of these structures is a key factor in terpene biosynthesis. Growth regulators can influence formation and development of essential oil biosynthesis and storage structures. The effect of plant hormone application on secretory structure formation was observed during a study of cytokinin's effect on Thimus mastichina essential oil production. In this work Fraternale and others (2003) verified higher yield of essential oil in the medium culture with benziladenine (BA) (10) at a concentration of 0.1 mg L-1. In leaves of T. mastichina plants treated with BA there was a larger density of glandular hair in post secretory stage. Although a direct correlation has not been demonstrated, the authors suggest an effect of BA on glandular hair development. In Norway spruce (Picea abies) jasmonate application altered plant anatomy raising the number of resiniferous ducts, and associated with its application a threefold increase in monoterpene concentrations like α- e β-pinene and limonene was observed (Erbilgin et al. 2006). In lavender (Lavandula dentata) a higher amount of leaves in plants cultured in vitro for 8 weeks with 0.1 mg L-1 (BA) was verified. The number of glands on the surface of leaves treated with BA was smaller, however they are not disrupted. In addition, the leaves showed an intense green color and remained young for a longer time. The observed effect was associated with leaf senescence delay and secretory gland differentiation, which keep them in the pre-secretory stage. Auxin (IBA) (11) was also used. Likewise to BA, plants treated with IBA presented a small number of glands, but were disrupted (post secretory stage), indicating that auxin accelerated their differentiation (Sudriá et al. 2004).
The action associated with essential oil biosynthesis in various steps of metabolic pathways is also one of the manners through which growth regulators could affect essential oil production.
Methyl-jasmonate applications (0.5 Mm) increased significantly the quantity of monoterpenes in basil (Ocimum basilicum). The content of terpenes in plants treated with methyl-jasmonate was higher than that found in control plants. The increase in eugenol and L-linalool compared to control plants was 56% and 43%, respectively. The authors noted an increment in phenylpropanoid pathway products derived from phenylalanine ammonia-lyase (PAL), as well as an increase in the number of transcripts of the enzymes present in subsequent steps of the pathway, which explains the eugenol increase. The effect of methyl-jasmonate application on enzymes associated with monoterpenoid biosynthesis was not verified. This result indicates that exogenous applications of that hormone can influence the production of compounds present in basil essential oil by gene regulation, promoting an increase in the number of transcripts of the enzymes linked to metabolic pathway of those compounds (Kim et al. 2006; Li et al. 2007). Rodriguez-Saona et al. (2001) observed that cotton plants (Gossypium hirsutum L.) treated with MeJ emitted a great amount of inducible volatiles such as linalool and β-ocimene. According to the authors, this result indicates that MeJ activates enzymes associated with the biosynthesis of those compounds. Zhang et al. (2005) verified that after GA application (14 µM) there was a 400% increase in the concentration of artemisinin compared to control plants. Treatment with GA3 does not correspond to an increase in amorpha-4,11-diene synthase, which catalyzes the first step in artemisinin biosynthesis. The authors suggest activation of artemisinic acid to artemisinin conversion as mechanism by which gibberellins increase artemisinin concentrations. Application of methyl-jasmonate (50 µL) resulted in a rise of ten fold in emission of volatile compounds in Iva frutescens compared to control plants, producing an increase of 14, 5 and 8-fold on α-pinene, sabinene and limonene emission, respectively. According to the authors, the increase in volatile emissions treated with methyl-jasmonate could be a result of terpene syntase activation and de novo synthesis. Methyl-jasmonate activates a range of pathways in Iva frutescens like shykimate, octadenoid, mevalonate and methylerythritol-4 phosphate (Degenhardt and Lincoln 2006). In Chrysanthemum cinerariaefolium piretrin production increased 31 and 44% in relation to control for 50 and 100 mg L-1 ethrel concentrations, respectively. It was also observed that under ethrel treatment there was a higher incorporation of 14CO2 in piretrins. According to the authors this result indicates that ethrel could influence the activity of enzymes linked to the piretrin biosynthetic pathway (Haque et al. 2007). In Catharanthus roseus cell suspension, Decendit et al. (1993) and Papon et al. (2005) observed an increase in geranyl 10-hidroxylase activity when the medium was supplied with cytokinin (zeatin). This enzyme acts in the terpene moiety of indolic alkaloids as ajmalicine, serpentine and catharantine. Oudin et al. (2007) verified that adding cytokinin to the medium increased the production of alkaloids with terpenic moiety. In in vitro culture of Lavandula dentata L. application of cytokinin (0.1 mg L-1 BA) had a positive effect on production and/or accumulation of essential oil. The increase was 150% related to control. Essential oil chemical composition was altered. The majority of compounds (1,8-cineole, fenchole, camphor and borneol) were maintained, they represent 80% of total chemicals in essential oil. Nevertheless, cytokinin application increased camphor percentage and decreased 1,8-cineole, while other compounds didn't show alteration. Cytokinin treatment produced a 140% increase in HMG-CoA redutase activity, yet this was not directly linked to a rise in oil production, but is connected to whole plant metabolism, which demands a supply of products from the pathways where HMG-CoA redutase acts to primary metabolism. In relation to chemical composition alteration, an influence of growth regulator on enzyme activities could be the responsible factor (Sudriá et al. 1999). In Salvia officinalis and M. piperita plants, enzymes that participate in the synthesis of compounds present in essential oil were extracted after treatment with 10 ppm of diphenylurea. It was observed that the activities of analyzed enzymes (bornyl pyrophosphate cyclase from S. officinalis and limonene cyclase from M. piperita) were higher in plants treated with hormone. Thus, the authors demonstrated that cytokinin foliar application stimulated essential oil accumulation, at least due to the direct effect on metabolism of monoterpenoids (El-Keltawi and Croteau 1987).
Concentration and source of the growth regulators applied are factors that can result in different responses.
El-Keltawi and Croteau (1987) applied different cytokinin sources (cinetin, diphenylurea (12), benzylaminopurine (13) and zeatin (14)) on concentrations from 1 to 10 ppm in species of Lamiaceae (Mentha piperita, M. spicata, M. suaveolens, Salvia officinalis e Lavandula vera), and verified that cinetin and diphenylurea were the most effective in increasing essential oil production. Although alterations on oxygenated monoterpenes have been detected, cytokinins did not drastically change essential oil composition of the studied species. There was reduction in content of some chemicals, nevertheless the absolute levels increased. According to the authors, the primary effect of cytokinins was a stimulus of monoterpene accumulation. The cinetin and diphenylurea effects were higher than that attributed to the effects related to growth and developmental changes, or on gland formation and density, thus an effect on metabolism was suggested.
In a study about the effects of different cytokinin sources like benzylaminopurine, cinetin and N6-isopenteniladenine on monoterpene biosynthesis in Cymbopogon species, Craveiro et al. (1989) verified an increase of 9 and 93% on the content of essential oil in C. citratus when treated with benzylaminopurine and N6-isopenteniladenine, respectively. Otherwise cinetin decreased essential oil content by 19%.
Just as different species can present variable responses to plant hormone application, one species could respond in different ways according to its development stage and number or interval of applications.
Exogenous application of cytokinin (BAP - 50 mg L-1) on Mentha piperita L., at 15 and 30 days after the beginning of the experiment, and with harvest at 45 days resulted in an increase of plant dry mass. The time of application didn't influence oil yield, but changed its chemical composition (Scravoni et al. 2006).
Application of cinetin in Rosa damascena demonstrated that the concentration of 5 mg L-1 raises citronellal and geranyl acetate production by 8% in the first year of application and 20% in the second year Farooqi et al. (1993). Using the same source at the concentration of 20 mg L-1, the increase was 13 and 24%, in the first and second year, respectively.
Figueiredo et al. (2006) analyzed the effect of application of hormones such as gibberellins and analogues of plant hormones like ethrel in Cymbopogon citratus at different times in a year and did not verify the effect of hormones on essential oil production.
Not only quantity, but also quality of essential oil can be influenced by growth regulator application.
Arikat et al. (2004), comparing production and chemical composition of sage (Salvia fruticosa Mill.) essential oil, observed that plants grown in vitro showed a high content of essential oil (0.7%) when compared to plants grown in a greenhouse (0.34%). They also verified that the main compounds (α-pinene, 1,8-cineole, camphor and borneol) were the same under both conditions. However, the percentages of camphor and borneol were expressively higher in plants grown in vitro. The authors report that essential oil percentages in plants grown in vitro are often higher to those found in plants from a greenhouse, associating this result with the presence of growth regulators, especially cytokinins.
In Salvia officinalis L. the chemical composition changed with gibberellic acid (100 mg L-1) application, with a significant reduction of β-tujone and α-humulene in relation to the control plants (Povh and Ono 2007).
Stoeva and Iliev (1997) applying citokinins (4PU-30 (15)- 25 and 50 mg L-1; DROPP (16)- 50 and 100 mg L-1) in mint (Mentha spicata (L.) Huds. cv. CS-87) verified changes in the chemical composition of essential oil, where 1,8-cineole presented an increase and carvone was reduced.
In Melissa officinalis grown in culture medium for 60 days with auxin and cytokinin complement (IAA 11.42 µmol L-1; BA 8.87 µmol L-1 and IAA+BA), an increase of 1.4 fold on nerol and 4.1 fold on geraniol was verified. Plants grown on control medium and ex vitro, however, presented higher percentages of neral and geranial. According to the authors, the hormones added to culture medium could have inhibited reactions of reduction from alcohol to aldehydes (Silva et al. 2005).
I found a chart that listed each terpenoid, and what flavor/aroma they are responsible for.
Myrcene is the most prevalent terpene found in most varieties of marijuana but not found in hemp. It is also present in high amounts in hops, lemon grass, East Indian bay tree, verbena and the plant from which it derives its name mercia. Myrcene appears in small amounts in the essential oils of many other plants.
Its odor is variously described as clove like, earthy, green-vegatative, citrus, fruity with tropical mango and minty nuances(In fact, myrcene is found in large qauntities in cavalo, rosa, espada, and paulista mangos). The various odors are the result of slight differences in the overall esential oil makeup. All of these flavors and odors are commonly used to describe Cannabis.
Myrcene is a potent analgesic, anti-inflammatory and antibiotic. It blocks the actions of cytochrome, aflatoxin B and other pro-mutagens that are implicated in carcinogenesis. It is present in small amounts in many essential oils associated with anti-depressive and uplifting behavior.
Myrcene is probably a synergist of THC: A combination of the two molecules creates a stronger experience than THC alone. Myrcene probably affects the permiability of the cell membranes, thus it may allow more THC to reach brain cells.
LIMONENE is found in the rinds of citrus and many other fruits and flowers. It is the second, third or fourth most prevalent terpene in cannabis resins. Everyone is familiar with the odor of citrus resins. They explode into the air when a fruit is peeled. The exact order is determined by the structure of the terpene.
Limonene has anti-bacterial, anti-fungal and anti cancer activities. It inhibits the ras cancer gene cascade, which promotes tumor growth. It is used to synergistically promote the absorbtion of other terpenes by penetrating cell membranes. Limonene sprays are also used to treat depression.
Since Limonene is such a potent anti-fungal and anti-cancer agent, it is thought to protect against aspergillus fungi and carcinogens found in cannabis smoke streams
.
Plants use Limonene to repulse predators. For instance, flies have a group of receptors similar in function to the taste buds on our tongues. One of them detects noxious chemicals, and responds to Limonene as if it were toxic. This is hard wired into the flies brain.
In humans, Limonene's design facilitates a direct response by quickly permeating the blood-brain barrier. The result is increased systolic blood pressure. One test, reported subjective alertness and restlessness. Various Limonene analogs can cue the brain to sexuality, buoyancy, or focused attention.
Caryophylene is a major terpene found in black pepper(15-25%), clove(10-20%) and cotton(15-25%). It is found in smaller %'s in many other herbs, and spices. It has a sweet, woody and dry clove odor and tastes pepper spicy with camphor and astringent citrus backgrounds. It contributes to black pepper's spiciness. The oil is used industrially to enhance tobacco flavor.
Caryophylene, given in high amounts, is a calcium and potassium ion channel blocker. As a result, it impedes the pressure excerted by heart muscles. As a topical it is analgesic and is one of the active constituents that makes clove oil, a preferred treatment for toothache.
It does not seam to be involved in mood change.
Pinene is the familiar odor associated with pine trees and their resins. It is the major component in turpentine and is found in many other plant essential oils in noticeable amounts including rosemary, sage, and eucalyptus. Many additional plant oils contain pinene.
Pinene is used medically as an expectorant, and topical antiseptic. It easily crosses the blood-brain barrier where it acts as a acetylcholinesterase inhibitor; that is, it inhibits activity of a chemical that destroys an information transfer molecule. This results in better memory. Largely due to the presence of pinene, rosemary and sage are both considered "memory plants."
Concoctions made from their leaves have been used for thousands of years in traditional medicine to retain and restore memory.
Pinene probably gives true skunk varieties, the ones that stink like the animal, much of their odor. It is also a bronchodilator. The smoke seems to expand in your lungs and the high comes on very quickly since a high percentage of the substance will pass into the bloodstream and brain. It also increases focus, self satisfaction and energy, which seems counterintuitive, but for the presence of terpineol.
TERPINEOL has a lilac, citrus or apple blossom/lime odor. It is a minor constituent of many plant essential oils. It is used in perfumes and soaps for fragrance.
Terpineol is obtained commercially from processing other turpines. It reduces motillity- the capability for movement- by 45% in lab rat tests. This may account for the couchlock effects of some cannabis although that odor is not usually associated with body highs. However, Terpineol is often found in cannabis with high pinene levels. Its odor would be masked by the pungent woodsy aromas of pinene.
BORNEOL smells much like the menthol aroma of camphor and is easily converted into it. It is found in small quantities in many essential oils. Comercially it is derived from artemisia plants such as wormwood and some species of cinnamon.
It is considered a "calming sedative" in chinese medicine. It is directed for fatigue, recovery from illness and stress.
The camphor like overtones of Silver Haze varieties are unmistakable. The high does have a calming effect as well as its psychedelic aspects. This probably means there is a large amounts of borneol present.
DELTA 3-CARENE has a sweet pungent odor. It is a constituent of pine and cedar resin but is found in many other plants including rosemary. In aroma therapy, cypress oil, high in D-3-carene, is used to dry excess fluids, tears, running noses, excess menstrual flow and perspiration. It may contribute to the dry eye and mouth experienced by some marijuana users.
LINALOOL has a floral scent reminiscent of spring flowers such as lily of the valley, but with spicy overtones. It is refined from lavender, neroli, and other essential oils. Humans can detect its odor at rates as low as one part per million in the air.
Linalool is being tested now for treatment of several types of cancer. It is also a component of several sedating essential oils. In tests on humans who inhaled it, it caused severe sedation. In tests on lab rats it reduced there activity by almost 75%.
PULEGONE has a minty-camphor odor and flavor that is used in the candy industry. It is implicated in liver damage in very high dosages. It is found in tiny quantities in marijuana.
Pulegone is an acetylcholinesterase inhibitor. That is, it stops the action of the protein that destroys acetylcholine, which is used by the brain to store memories. It may counteract THC's activity, which leads to low acetylcholine levels. The result is you would forget more on THC alone than THC accompanied by pulegone.
1,8-CINEOLE is the main ingredient in oil of eucalyptus. It has camphor-minty odor. It is also found in other fragrant plants and in minor amounts in marijuana. It is used to increase circulation, pain relief and has other topical uses.
Cineole easily crosses the blood-brain-barrier and triggers a fast olfactory reaction. Eucalyptus oil is considered centering, balancing and stimulating. It is probably the stimulating and thought provoking part of the cannabis smoke stream.
There are 100+ chemicals It's not just about THC
Marijuana is a mix of many different compounds. Most of them fall into three categories: cannabinoids, terpenoids, and flavanoids. Each of these compounds can significantly modify the therapeutic benefits of the plant. There are hundreds of different chemical combinations each producing a different effect. Some of these effects and interactions still have yet to be discovered!
View Image
http://skunkpharmresearch.com/cannabinoid-info/
Possible site of cannabinoid synthesis
Our studies contribute several pieces to the puzzle on where cannabinoids are synthesized, yet we lack definitive information on the precise site of their formation. Data from the antibody probe showing that they are not evident in the cytoplasm of the disc cells suggest that they may be formed at or outside the plasma membrane surface. A working model for continued study on their synthesis is embodied in diagram 3.
View Image
Diagram 3. (Left) Representation of gland illustrating the possible process in cannabinoid localization in secretory cavity. A phenol glucoside is transported into a disc cell and stored as free phenol in the vacuole. Terpene is synthesized by the specialized plastid, lipoplast, in disc cells. These precursors, terpene and phenol, react to form cannabinoids at the plasma membrane surface or in the wall whereupon they appear in the secretory cavity.
As described in the cannabinoid pathway, these dimeric compounds consist of terpene and phenolic components. The abundant secretory activity of the disc cell plastids, and knowledge that this organelle does synthesize terpenes, suggests that they contribute the terpene component. Our detection, in previous studies, of abundant phenol in whole glands, and knowledge that phenols accumulate in vacuoles of cells, suggests that this cell feature may contribute the phenol component. Phenols are transported in the plant as glycosides and, when becoming localized in a cell vacuole, they accumulate there upon dissociation of the sugar moiety which returns to the cell cytoplasm.
We hypothesize that terpenes and phenols, when released from their respective sources, accumulate at the plasma membrane and cell wall interphase where enzymes dimerize these compounds into cannabinoids. It is necessary to determine enzymes involved in cannabinoid synthesis. Such an enzyme, when available, can be prepared as an antibody probe that can be used to identify more precisely the locus of cannabinoid, and THC, synthesis. Glands represent unique structures, and can be utilized to broaden our understanding of cannabinoid synthesis and aid in our effort to reduce the cannabinoid content of Cannabis strains for production of industrial hemp.
CONCLUSIONS
1. THC accumulated in abundance in the secretory cavity where it was associated with the: a) cell walls, b) surface feature of secretory vesicles, c) fibrillar material released from disc cell wall, and d) cuticle. It was not associated with the content of the secretory vesicles. The association of THC with structural components, particularly the wall, fibrillar matrix and surface feature of vesicles, suggests that it may be chemically bound to them rather than being free in the cavity. If THC and other cannabinoids are bound to components in the cavity, their presence and movement may require a source of energy in the cavity. Additional studies are necessary to determine their bound or free status.
2. Little or no THC was detected in the cytoplasm of the disc cells. This suggests that the terpene and phenol precursors, which must occur in the disc cells at some interval, may form the cannabinoids at the surface of the plasma membrane, or in the cell wall facing the secretory cavity.
3. Some THC was present in the cell walls of other cells. Genes for cannabinoid synthesis are present in all cells of the plant, but tissues other than glands produce low levels of these compounds.
4. Reduction or elimination of glands by mutation procedures will reduce significantly the quantity of THC in the plant. However, the pathway for cannabinoid synthesis is controlled genetically: glands are specialized to synthesize high levels of cannabinoids. Thus, a glandless plant can be expected to synthesize very low levels of cannabinoids. We do not know the roles of cannabinoids in the glands. They may be involved in some way with "protection", or other role. The absence of glands may or may not alter the functional role. Since other cells also synthesize these compounds, at very low levels, the quantity may be sufficient to perform the functional role. Therefore, a glandless mutant(s) would serve our purpose to reduce the THC concentration for utilization of such strains in the hemp industry.
UV Light and Terpenoids
https://www.icmag.com/ic/showthread.php?t=139726
View Image
View Image
hey shaggy
had to bump this
waay to much good info to sit on the bottom of the heap
A note on just a tiny portion of the above post.
Heat evaporates the terpines.
Before global warming Alaska would have about three weeks per year of minus forty or colder. During these spells I would take a few gallons of butane outside and do an overnight run of extactions.
Butane stays liquid under -11 so our distillery rig was used with a brine solution maintained at zero degrees F, a nice slow boil for the butane. The actual agitation of the mix was 45 seconds, THC has the viscosity of alcohol and remains liquid at -40 while most other plant oils have congealed into waxes. The quick rinse leaves most contaminants behind.
The resulting solution was boilled down at zero degrees and the final purge was done with vacuum at room temperature resulting in a very clean product.
I cannot really compare the cold weather product with the hexane extracts currently being done. Even with vacuum the boiling temperature approximates 140 degrees F with hexane solvent. The final product is 60% THC but almost no terpines at all.
The outdoor extract had the same relative amount of THC but the other forty percent is mostly terpines.
I miss the cold weather, nothing I have found on the market today can compare, or even come remotely close to the effects of cold processed bud extract.
Heat evaporates the terpines.
Before global warming Alaska would have about three weeks per year of minus forty or colder. During these spells I would take a few gallons of butane outside and do an overnight run of extactions.
Butane stays liquid under -11 so our distillery rig was used with a brine solution maintained at zero degrees F, a nice slow boil for the butane. The actual agitation of the mix was 45 seconds, THC has the viscosity of alcohol and remains liquid at -40 while most other plant oils have congealed into waxes. The quick rinse leaves most contaminants behind.
The resulting solution was boilled down at zero degrees and the final purge was done with vacuum at room temperature resulting in a very clean product.
I cannot really compare the cold weather product with the hexane extracts currently being done. Even with vacuum the boiling temperature approximates 140 degrees F with hexane solvent. The final product is 60% THC but almost no terpines at all.
The outdoor extract had the same relative amount of THC but the other forty percent is mostly terpines.
I miss the cold weather, nothing I have found on the market today can compare, or even come remotely close to the effects of cold processed bud extract.
superglue
Member
A note on just a tiny portion of the above post.
Heat evaporates the terpines.
Before global warming Alaska would have about three weeks per year of minus forty or colder. During these spells I would take a few gallons of butane outside and do an overnight run of extactions.
Butane stays liquid under -11 so our distillery rig was used with a brine solution maintained at zero degrees F, a nice slow boil for the butane. The actual agitation of the mix was 45 seconds, THC has the viscosity of alcohol and remains liquid at -40 while most other plant oils have congealed into waxes. The quick rinse leaves most contaminants behind.
The resulting solution was boilled down at zero degrees and the final purge was done with vacuum at room temperature resulting in a very clean product.
I cannot really compare the cold weather product with the hexane extracts currently being done. Even with vacuum the boiling temperature approximates 140 degrees F with hexane solvent. The final product is 60% THC but almost no terpines at all.
The outdoor extract had the same relative amount of THC but the other forty percent is mostly terpines.
I miss the cold weather, nothing I have found on the market today can compare, or even come remotely close to the effects of cold processed bud extract.
hey Phaeton
that there is a cool story no pun intended
down here in washington.. we would get a couple week cold spell (sub 32f) and i would process all my bubble hash at that time
a line in your response caught my eye
" THC has the viscosity of alcohol and remains liquid at -40"
i think you may have been looking at a combination of monoterps with the thc as the mono terps are viscous like ethanol at low temp and thc would be glass hard at -40f..or even 0f from my experience
something to think about
hey Phaeton
that there is a cool story no pun intended
down here in washington.. we would get a couple week cold spell (sub 32f) and i would process all my bubble hash at that time
a line in your response caught my eye
" THC has the viscosity of alcohol and remains liquid at -40"
i think you may have been looking at a combination of monoterps with the thc as the mono terps are viscous like ethanol at low temp and thc would be glass hard at -40f..or even 0f from my experience
something to think about
There is the possibility the study I used confused the chemicals being analyzed.
The results from a 45 second agitation at -40 versus a 45 second agitation at room temperature resulted in little change in THC content and a very large change in the contaminant content. Roughly 95% THC and 40% less total weight at -40.
A rousing oil business was taking advantage of a fad that lasted two years. Summer and winter records were compared.
These empirical results lend credence the the conclusions drawn from the fractional distillation separation done in the study. The THC remains solvent at -40 while other non polar resins and oils become more impervious. Since I can no longer reference the original paper this is an anecdote of how the extractions worked when I did them.
I remember summertime processing of bubblehash, the trunk of the car would have a hundred pounds of bag ice from the local 7-11 store.
Winters are good for many things, within limits.



