-
ICMag with help from Landrace Warden and The Vault is running a NEW contest in November! You can check it here. Prizes are seeds & forum premium access. Come join in!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
It is a good chart to note but I think it also could lead one in a wrong direction - using much of ph ups and downs. My view on this is that instead of concentrating on fighting to perfect PH it is much better just to make sure it stays in somewhat acceptable range (easily done using some lime) and let benefitials to do their job. Like miccorhizae.
G
Guest
Headypete - being an organic grower, you're probably not going to want to hear chemistry set advice. LOL
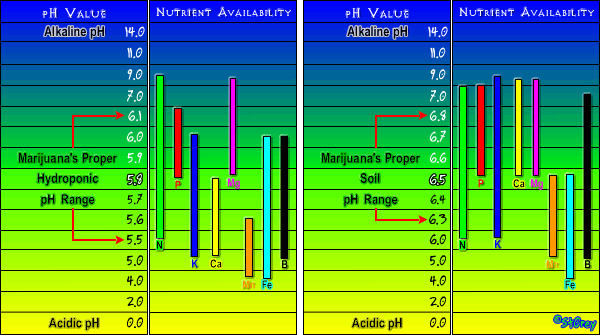
The plant takes up nutrients using a chemical reaction between the roots, soil and water. The nutrients, minerals and salts bind to the soil medium because they are ionically charged. The plant is able to take up some easier than others. This is where the pH comes into play. By the chart, 5.8 is optimum for hydro and 6.5 is optimum for soil.
By the way, soil is easier to grow in because the optimum growing range is much wider. The Major nutes are N, P, K with minor nutes Ca and Mg being very important to the plant.
One thing to be aware of is that adding nutes generally lowers the pH so low pH indicates overfeeding and a buildup of nutrients in the soil.
So it's the soil vs. water that makes the difference in ph ranges? Is the higher ph need to free the nutrients bound with soil particles? How does the differing ph come into play?
What is the scientific reason/mechanics that call for two different ph ranges?
Thanks for the info RG. I love to find out answers to questions, chemistry or whatever! It all helps me to be a better, more knowledgeable grower.
What is the scientific reason/mechanics that call for two different ph ranges?
Thanks for the info RG. I love to find out answers to questions, chemistry or whatever! It all helps me to be a better, more knowledgeable grower.
G
Guest
Lucas has a pretty decent explanation here if you can follow the chemistry.
http://www.icmag.com/ic/showthread.php?t=21119
Basically the roots produce H+ ions to absorb cations Ammonium NH+, Potassium K+, Calcium++ and Magnesium++. The buildup of H+ ions over time lowers the pH.
The plant will absorb anions in the form of nitrates and phosphates which leaves hydroxyls OH- and bicarbonates that will react with the H+ ions to form water H+ and OH- => H20
All I can say is the plant takes up some ions better than others because they all have different charges and the pH comes into play, very much in Hydro than in Soil.
One other thing I've found out is that if you just feed on every watering, the high nutrient concentration may lock out some of the weaker nutes. A weekly feeding strategy with a plain watering in between ensures that the weaker nutes get absorbed.
http://www.icmag.com/ic/showthread.php?t=21119
Basically the roots produce H+ ions to absorb cations Ammonium NH+, Potassium K+, Calcium++ and Magnesium++. The buildup of H+ ions over time lowers the pH.
The plant will absorb anions in the form of nitrates and phosphates which leaves hydroxyls OH- and bicarbonates that will react with the H+ ions to form water H+ and OH- => H20
All I can say is the plant takes up some ions better than others because they all have different charges and the pH comes into play, very much in Hydro than in Soil.
One other thing I've found out is that if you just feed on every watering, the high nutrient concentration may lock out some of the weaker nutes. A weekly feeding strategy with a plain watering in between ensures that the weaker nutes get absorbed.
Country Mon
Active member
Hey, guys -
Great info. I have been mulling over ph lately. I shoot for 6.5 in soil, with the reasoning (true or not) that the ph will slide down a bit over the 3-4 days between waterings.
My big question for everybody in the know is this: how much ph up is too much? I never have the need to use ph down; I'm always moving my solution up from the high 4s to the low 5s to 6.5 - 6.8. I have r/o water that is a steady 7.
And a better question: what is a good brand of ph up that I can use as much as I need of to to hit my 6.5 - 6.8 without causing problems? Earth Juice? I used to use a hydrated-lime-in-water product that was great, but the company stopped making it. Maybe I should attempt my own hydrated lime ph up?
I'm pulling a steady 3/4 gram a watt every 8 weeks, so I can't be doing too much damage. But I'm always looking to improve.
Great info. I have been mulling over ph lately. I shoot for 6.5 in soil, with the reasoning (true or not) that the ph will slide down a bit over the 3-4 days between waterings.
My big question for everybody in the know is this: how much ph up is too much? I never have the need to use ph down; I'm always moving my solution up from the high 4s to the low 5s to 6.5 - 6.8. I have r/o water that is a steady 7.
And a better question: what is a good brand of ph up that I can use as much as I need of to to hit my 6.5 - 6.8 without causing problems? Earth Juice? I used to use a hydrated-lime-in-water product that was great, but the company stopped making it. Maybe I should attempt my own hydrated lime ph up?
I'm pulling a steady 3/4 gram a watt every 8 weeks, so I can't be doing too much damage. But I'm always looking to improve.
RealSupreme
Member
great info!
Crhazey Daze
Member
Ty-Stik said:Kinda of reminds me of a first period carpenters apprentice some years ago that couldn't drill the holes in a piece of wood. It wasn't a dull drill bit that was the problem, he just didn't understand that he had the drill motor running in reverse. There is a difference.
That is completely something i would do! To a T!


The _Selecta
Member
larry , im really curious as to why you would be using h202 , you stated being in soil (foxfarm) correct ? which foxfarm is it, ocean forest , light warrior? the application of h202 seems to be far advanced for use with these techniques, i believe its only beneficial to use h202 singularly and also only in the night cycle . therefore IMO it would seem that by you adding the h202 to your pretty much cancelling out any option of chelating. all those micros must be sad little buggers..also i believe that foxfarm soils have ph buffer already in them be lime or shells? this is all my assumption larry , strictly from the little knowledge i have taken in about h202 , im very interested in using h202 and would love some more educated insight, maybe someone more educated will chime in... 

Last edited:
Crhazey Daze
Member
HeadyPete said:Stop the epsom salts and hydrogen peroxide...
PH naturally drops from microbial action, which you don't have cause the hydrogen peroxide killed all your beneficial organisms. Beneficials consume the nutrients you add, excrete waste and this waste is what the plant consumes as nutrients. This is only for organic ferts, mineral or synthetic ferts are taken up directly by the plant. With no beneficials, your plants are not getting food. The nutes you add just get washed out.
I don't understand how hydrogen peroxide of 3% dilution is going to harm beneficials?
It almost immediately decomposes into water and oxygen.
Unless you can explain it in an intelligent manner, perhaps you should keep your wise ass comments to yourself, asshole. I highly doubt you have even close to the mental capacity to match wits with me.
Richard Cranium...is that you?
Richard Cranium...is that you?
G
Guest
hoosierdaddy said:Unless you can explain it in an intelligent manner, perhaps you should keep your wise ass comments to yourself, asshole. I highly doubt you have even close to the mental capacity to match wits with me.
Richard Cranium...is that you?
Hydrogen Peroxide is a powerful "oxidizer". And just as it oxidizes the harmful bacteria in a cut on your hand, it will oxidize the beneficial bacteria in your soil....and it will do it before it breaks down.
pedro

Yes, but at what concentration?
H2O2 is also used as a rocket propellant at levels above ~90%
H2O2 is also used as a rocket propellant at levels above ~90%
G
Guest
hoosierdaddy said:Yes, but at what concentration?
H2O2 is also used as a rocket propellant at levels above ~90%
It will oxidize the bacteria at very low concentrations. The folks above are correct. H2O2 should not be put into organic soils.
Why would you want to do that? If you want more dissolved O2 in your water, bubble it, ...andcold water will hold more dissolved O2.
pedro

It's not that I want to do this, per se...it's just that from my searching, I have found that gardeners of all flavors have found the addition of H2O2 to be a very beneficial add.
And like many things, internet arguments ensue when a topic that is not fully understood by all gets discussed. This often times leads to unwarranted warnings by those who have few clues, and simply want to appear learned.
And even botanists seem to have some disagreement on this particular topic, so I have my doubts that internet bloggers have much more of an insight. Although, many may have some first hand experience that they can share, even if they do not fully understand the science and chemistry behind the effects.
From what I can gather, no amount of bubbling will create the free radical oxygen molecule that H2O2 can. And it is this molecule that is what helps the plant uptake better, from what I can find out.
And yes, perhaps if we are growing in hydro, and have added probiotics, then it may not be a good idea to work the other direction by taking the chance of H2O2 harming them. But in soil, IMO, from what I have read and seen, that the benefits of a H2O2 solution, in the appropriate dilution, can far outweigh any sort of presumed probiotic harm.
And what if we choose to use H2O2 as a foliage feed?
I can tell you from first hand experience that an addition of H2O2 can absolutely increases healthy growth. But I have only experimented with various dilutions using 3% H2O2, and have never tried using hydro store snake oil at 33-35% concentrations. I suspect, since many seem to be jack batty over adding this and that, in a multitude of flavors and concoctions, that some may have seen some bad results using hydro store crap (including the recommendations of these hydro store numbskulls).
And like many things, internet arguments ensue when a topic that is not fully understood by all gets discussed. This often times leads to unwarranted warnings by those who have few clues, and simply want to appear learned.
And even botanists seem to have some disagreement on this particular topic, so I have my doubts that internet bloggers have much more of an insight. Although, many may have some first hand experience that they can share, even if they do not fully understand the science and chemistry behind the effects.
From what I can gather, no amount of bubbling will create the free radical oxygen molecule that H2O2 can. And it is this molecule that is what helps the plant uptake better, from what I can find out.
And yes, perhaps if we are growing in hydro, and have added probiotics, then it may not be a good idea to work the other direction by taking the chance of H2O2 harming them. But in soil, IMO, from what I have read and seen, that the benefits of a H2O2 solution, in the appropriate dilution, can far outweigh any sort of presumed probiotic harm.
And what if we choose to use H2O2 as a foliage feed?
I can tell you from first hand experience that an addition of H2O2 can absolutely increases healthy growth. But I have only experimented with various dilutions using 3% H2O2, and have never tried using hydro store snake oil at 33-35% concentrations. I suspect, since many seem to be jack batty over adding this and that, in a multitude of flavors and concoctions, that some may have seen some bad results using hydro store crap (including the recommendations of these hydro store numbskulls).



