-
As of today ICMag has his own Discord server. In this Discord server you can chat, talk with eachother, listen to music, share stories and pictures...and much more. Join now and let's grow together! Join ICMag Discord here! More details in this thread here: here.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Profile that would most likely contribute to trippy strains and conditions.
- Thread starter Verdant Whisperer
- Start date
Verdant Whisperer
Well-known member
It started with a month-long investigation into terpenes and possible connection to flowering times, then evolved into looking into other aspects of the plant, what i found is that while there is correlations of similiar terpenes in alot of long flowering sativa's they didn't really have an effect on flowering times, it was the hormone levels and their relationships to the terpenes what made me notice the correlation. not the terpenes themselves. I am not associated with a institution and i look up things and come up with conclusions in my own way. I paste certaint points and aspects of my investigation/research in word documents then look for correlations or sometimes an idea sparks my mind and i look into it or ill stumble across a small detail that turned out to be something bigger. then I take my ideas and collaborate and check them against AI, and when i've come up with my conclusion i have it made into an article format to save. I do not research in the same way as the articles in Skunksmans post as i am not a scientist/doctor and have no credentails, what i do have is an open and creative mind that see's things differently than most scientist, who are stuck behind the brick walls they build being indoctrinated into an institution not having the freedom of mind as someone whos always been a free thinker, never been a pack animal in a sense, more of a lone wolf. if you want to discredit my investigation because lack of scientific procedure thats ok, i can give supporting details to all the claims i make and why. I think its a good thing having the input of someone who has no bias, just seeking truth. in my theory on terpenes and flowering times i was wrong in a sense, but i still figured out why those terpenes where higher in those varities. and the hormonal differences in most NLD and SLD strains.What studies have you done, and how were they done? I am not discounting that terpenes play a role, but I consider one role among many, such as minor cannabinoids. I think it's extremely complex.
For example, a lot of the medical cannabis strains I use are high in caryophyllene which you stated should be trippy, yet the strains I get that are high in this are nothing of the sort.
Verdant Whisperer
Well-known member
So i found a strain in that thread with a profile known for being visual and euphoric (Sage) and it matches my description of a trippy strain as well as GDP for a grounded strain.
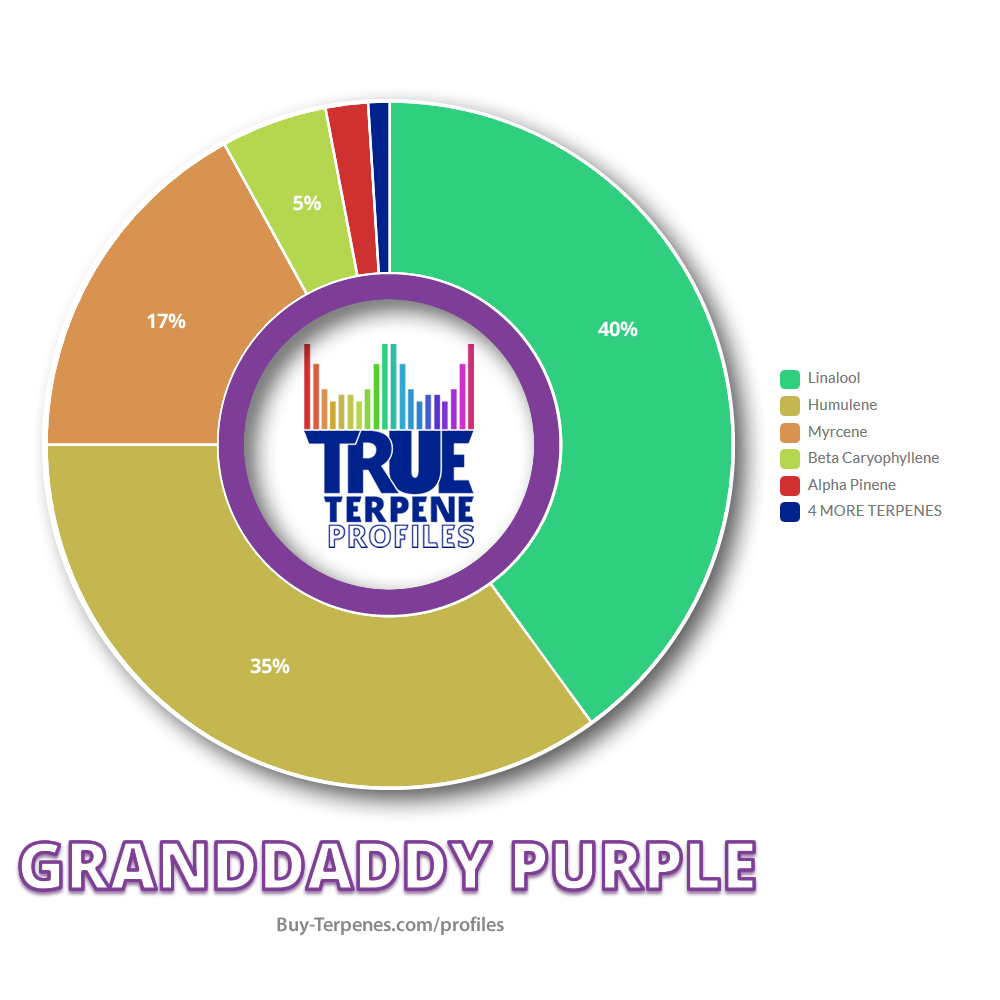
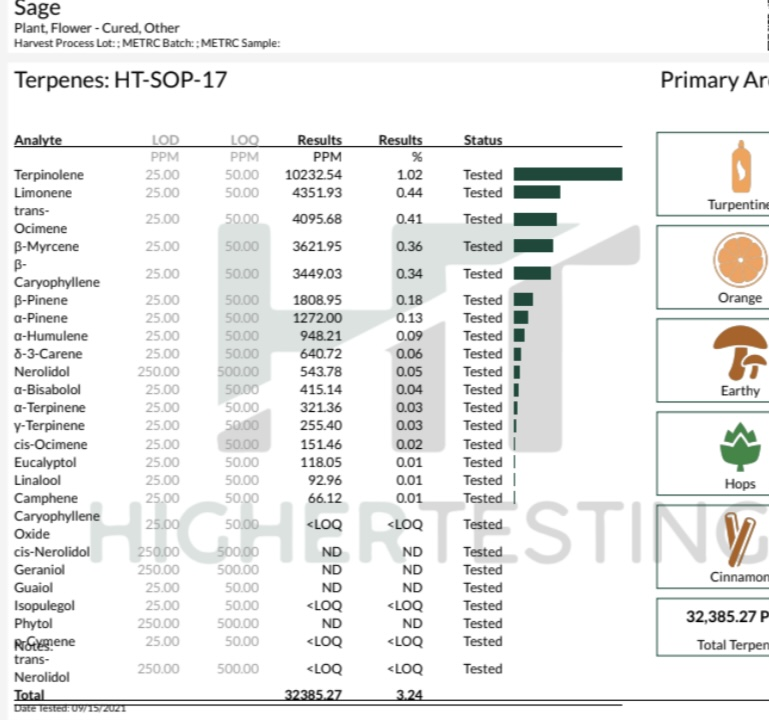
Last edited:
Do you mean the search for trip weed thread by Thai Bliss? It's a sticky in the strains and breeding and has recently begun again.Was a thread on Trippy Cannabis that ran for years until rather recently,
Might be a lot of that content would be of interest to you.
Verdant Whisperer
Well-known member
Do you mean the search for trip weed thread by Thai Bliss? It's a sticky in the strains and breeding and has recently begun again.
Cannabinols for trippy visuals
Karma talked about happy brothers strain and a terpine cannabinoid makeup that makes psychoactive compounds that make you see brighter colors visuals more defines lines and woke up clarity and energy im looking for a strain like this terpines aimed at this so the common mycrene caraphlene and...
www.icmag.com
I have no issue with people researching in this way, but I do need to point out that scientific research works to remove bias. Just because someone works in a scientific institution does not make them biased. Scientific method is about reducing variables and bias. That's simply the way it works.It started with a month-long investigation into terpenes and possible connection to flowering times, then evolved into looking into other aspects of the plant, what i found is that while there is correlations of similiar terpenes in alot of long flowering sativa's they didn't really have an effect on flowering times, it was the hormone levels and their relationships to the terpenes what made me notice the correlation. not the terpenes themselves. I am not associated with a institution and i look up things and come up with conclusions in my own way. I paste certaint points and aspects of my investigation/research in word documents then look for correlations or sometimes an idea sparks my mind and i look into it or ill stumble across a small detail that turned out to be something bigger. then I take my ideas and collaborate and check them against AI, and when i've come up with my conclusion i have it made into an article format to save. I do not research in the same way as the articles in Skunksmans post as i am not a scientist/doctor and have no credentails, what i do have is an open and creative mind that see's things differently than most scientist, who are stuck behind the brick walls they build being indoctrinated into an institution not having the freedom of mind as someone whos always been a free thinker, never been a pack animal in a sense, more of a lone wolf. if you want to discredit my investigation because lack of scientific procedure thats ok, i can give supporting details to all the claims i make and why. I think its a good thing having the input of someone who has no bias, just seeking truth. in my theory on terpenes and flowering times i was wrong in a sense, but i still figured out why those terpenes where higher in those varities. and the hormonal differences in most NLD and SLD strains.
That is indeed the thread that I was thinking of.Do you mean the search for trip weed thread by Thai Bliss? It's a sticky in the strains and breeding and has recently begun again.
Glad it made it as a sticky, and that it is still going,
Long admired that thread.
Verdant Whisperer
Well-known member
Thank you for correcting me, and I have no right to talk trash about people with years of experience and studies beyond mine. I've met super down to earth Ph'd and others who think they know everything and are close minded. but the ones ive met more open minded are less associated with institutions in general.I have no issue with people researching in this way, but I do need to point out that scientific research works to remove bias. Just because someone works in a scientific institution does not make them biased. Scientific method is about reducing variables and bias. That's simply the way it work.
Terpedia – The Terpene Encyclopedia
 terpedia.com
terpedia.com
IC Terpenes
A chemotaxonomic analysis of terpenoid variation in Cannabis
Karl W. Hillig
Biochemical Systematics and Ecology 32 (2004) 875–891
doi:10.1016/j.bse.2004.04.004
To determine whether the terpenoid composition of the essential oil of Cannabis is useful for chemotaxonomic discrimination, extracts of pistillate inflorescences of 162 greenhouse grown plants of diverse origin were analyzed by gas chromatography. Peak area ratios of 48 compounds were subjected to multivariate analysis and the results interpreted with respect to
geographic origin and taxonomic affiliation. A canonical analysis in which the plants were pre-assigned to C. sativa or C. indica based on previous genetic, morphological, and chemotaxonomic studies resulted in 91% correct assignment of the plants to their pre-assigned species. A scatterplot on the first two principal component axes shows that plants of accessions from Afghanistan assigned to the wide-leaflet drug biotype (an infraspecific taxon of unspecified rank) of C. indica group apart from the other putative taxa. The essential oil of these plants usually had relatively high ratios of guaiol, isomers of eudesmol, and other unidentified compounds. Plants assigned to the narrow-leaflet drug biotype of C. indica tended to have relatively high ratios of trans-b-farnesene. Cultivars of the two drug biotypes may exhibit distinctive medicinal properties due to significant differences in terpenoid composition.
A Green Extraction Process to Recover Polyphenols from Byproducts of Hemp Oil Processing.
Mourtzinos, I., Menexis, N., Iakovidis, D., Makris, D., & Goula, A.
Recycling, 3(2), 15.(2018).
doi:10.3390/recycling3020015
The valorization of solid waste hemp (Cannabis sativa L.) by a non-conventional method is presented in this article. Hemp polyphenols were extracted using aqueous solutions of 2-hydroxypropyl-β-cyclodextrin as an eco-friendly extraction solvent. Cyclodextrins (CD’s) are known to enhance the extraction of polyphenols in water by forming water soluble inclusion complexes. The process was optimized by implementing a response surface methodology (RSM) that took into consideration the following independent variables: CD concentration (CCD), solid-to-liquid ratio (S/L), and temperature (T). The assessment of the extraction model was based on two responses: the total polyphenol yield (YTP) and the antiradical activity (AAR). The optimum operating conditions were found to be: CD concentration, 32.1% (w/v); solid/solvent ratio, 1/15.2 g/mL; and extraction temperature, 28 ◦C. Different kinetic models were employed to fit with experimental data and the Peleg’s model was successfully developed for describing the mechanism of extraction under different processing parameters.
NOT CANNABIS SPECIFIC
A Heteromeric Membrane-Bound Prenyltransferase Complex from Hop Catalyzes Three Sequential Aromatic Prenylations in the Bitter Acid Pathway.
Li, H., Ban, Z., Qin, H., Ma, L., King, A. J., & Wang, G.
Plant Physiology, 167(3), 650–659. (2015).
doi:10.1104/pp.114.253682
Bitter acids (?-type and ?-type) account for more than 30% of the fresh weight of hop (Humulus lupulus L.) glandular trichomes and are well-known for their contribution to the bitter taste of beer. These multi-prenylated chemicals also show diverse biological activities, some of which have potential benefits to human health. The bitter acid biosynthetic pathway has been investigated extensively and the genes for the early steps of bitter acid synthesis have been cloned and functionally characterized. However, little is known about the enzyme(s) that catalyze three sequential prenylation steps in the ?-bitter acid pathway. Here, we employed a yeast system for the functional identification of aromatic prenyltransferase genes (PT). Two PT genes (HlPT1L and HlPT2) obtained from a hop trichome-specific cDNA library were functionally characterized using this yeast system. Coexpression of codon-optimized PT1L and PT2 in yeast, together with upstreamgenes, led to the production of bitter acids, but no bitter acids were detected when either of the PT genes was expressed by itself. Step-wise mutation of the Asp-rich motifs in PT1L and PT2 further revealed the prenylation sequence of these two enzymes in ?-bitter acid biosynthesis: PT1L only catalyzed the first prenylation step; PT2 catalyzed the two subsequent prenylation steps. A metabolon formed through interactions between PT1L and PT2 was demonstrated using a yeast two hybrid system, reciprocal co-immunoprecipitation, and in vitro biochemical assays. These results provide direct evidence of the involvement of a functional metabolon of membrane-bound prenyltransferases in bitter acid biosynthesis in hop.
A Systematic Approach to Developing Terpene Extraction Conditions Utilising Supercritical Carbon Dioxide
Eric Kawka
Chromatography Today Feb/March 2018
https://www.chromatographytoday.com/...-dioxidep/2337
Cannabis sativa plants produce and accumalate terpene-rich resin within the secretory cells of glandular trichomes. Monoterpenes and sesquiterpenes are important components of Cannabis resin as they contribute to the unique attributes of different Cannabis strains. Terpenes are responsible for the plants aroma and flavor.
Accelerated Solvent Extraction of Terpenes in Cannabis Coupled With Various Injection Techniques for GC-MS Analysis
Colton Myers, Jason S. Herrington, Paul Hamrah, Kelsey Anderson,
Frontiers in Chemistry 9 April 2021
DOI: 10.3389/fchem.2021.619770
https://www.researchgate.net/publica...GC-MS_Analysis
The cannabis market is expanding exponentially in the United States. As state-wide legalization increases, so do demands for analytical testing methodologies. One of the main tests conducted on cannabis products is the analysis for terpenes. This research focused on implementation of accelerated solvent extraction (ASE), utilizing surrogate matrix matching, and evaluation of traditional vs. more modern sample introduction techniques for analyzing terpenes via gas chromatography–mass spectrometry (GC-MS). Introduction techniques included Headspace-Syringe (HS-Syringe), HS-Solid Phase Microextraction Arrow (HS-SPME Arrow), Direct Immersion-SPME Arrow (DI-SPME Arrow), and Liquid Injection-Syringe (LI-Syringe). The LI-Syringe approach was deemed the most straightforward and robust method with terpene working ranges of 0.04–5.12 μg/mL; r ² values of 0.988–0.996 (0.993 average); limit of quantitation values of 0.017–0.129 μg/mL (0.047 average); analytical precisions of 2.58–9.64% RSD (1.56 average); overall ASE-LI-Syringe-GC-MS method precisions of 1.73–14.6% RSD (4.97 average); and % recoveries of 84.6–98.9% (90.2 average) for the 23 terpenes of interest. Sample workflows and results are discussed, with an evaluation of the advantages/limitations of each approach and opportunities for future work.
Accumulation of bioactive metabolites in cultivated medical Cannabis.
Richins, R. D., Rodriguez-Uribe, L., Lowe, K., Ferral, R., & O’Connell, M. A.
PLOS ONE, 13(7), e0201119.(2018).
doi:10.1371/journal.pone.0201119
There has been an increased use of medical Cannabis in the United States of America as more states legalize its use. Complete chemical analyses of this material can vary considerably between producers and is often not fully provided to consumers. As phytochemists in a state with legal medical Cannabis we sought to characterize the accumulation of phytochemicals in material grown by licensed commercial producers. We report the development of a simple extraction and analysis method, amenable to use by commercial laboratories for the detection and quantification of both cannabinoids and terpenoids. Through analysis of developing flowers on plants, we can identify sources of variability of floral metabolites due to flower maturity and position on the plant. The terpenoid composition varied by accession and was used to cluster cannabis strains into specific types. Inclusion of terpenoids with cannabinoids in the analysis of medical cannabis should be encouraged, as both of these classes of compounds could play a role in the beneficial medical effects of different cannabis strains.
ADDING TERPENES TO CONCENTRATES - THE SCIENCE AND THE EFFECTS
MMJDOCTORONLINE Staff
https://mmjdoctoronline.com/health-n...nd-the-effects
Before we get into all the wonderful medical and recreational aspects of terpenes, let's briefly describe what a terpene is. What are Terpenes and what is their effect in making Marijuana Tinctures?
The Terpenes are class of small multi-carbon chained molecules found everwhere in the plant kingdom. They give a straint its flavor, boquet its character. Terpene aroma is a means of communication to animals, insects, and other plants, that all have the ability to sense or smell these small molecules. Bees may be attracted to the flower full of pollen (and terpenes) while other insects avoid some types of terpenes, particularly those in bark, which are a deadly toxin.
Terpenes are seen as “balancers and communicators” in cannabis, where they can amplify, moderate or enhance the activity of THC and the other cannabinoids, by reacting with the CB1 and probably the CB2 receptors that effect many physical and psychological functions in the body.
Alkanes of the essential oil of Cannabis sativa.
Hendriks, H., Malingré, T. M., Batterman, S., & Bos, R.
Phytochemistry, 16(6), 719–721. (1977).
doi:10.1016/s0031-9422(00)89239-0
A waxy fraction obtained by column chromatography of the essential oil of Cannabis sativa consists of n-alkanes ranging from Cg to C,,, 2-methyl and 3-methyl alkanes and some dimethyl alkanes. The qualitative and quantitative composition of this fraction has been compared with the alkane fraction obtained by extraction of the herb.
All About Terpinene
Asia Mayfield
https://terpenesandtesting.com/all-about-terpinene/
γ-Terpinene and terpinolene in cannabis
Terpinene isomers may show up in various cultivars. For example, one popular dispensary reports that terpinolene can be found in cultivars including Jack Herer, Durban Poison, and Super Lemon Haze.
Not Cannabis specific
Alteration of product formation by directed mutagenesis and truncation of the multiple-product sesquiterpene synthases ?-selinene synthase and ?-humulene synthase
Dawn B Little, Rodney B Croteau
Archives of Biochemistry and Biophysics Volume 402, Issue 1, 2002, Pages 120-135
DOI: 10.1016/S0003-9861(02)00068-1
Two recombinant sesquiterpene synthases from grand fir, ?-selinene synthase and ?-humulene synthase, each produce more than 30 sesquiterpene olefins from the acyclic precursor farnesyl diphosphate. These enzymes contain a pair of DDxxD motifs, on opposite lips of the presumptive active site, which are thought to be involved in substrate binding and could promote multiple orientations of the substrate alkyl chain from which multiple families of cyclic olefins could derive.Mutagenesis of the first aspartate of either DDxxD motif resulted in depressed kcat, with lesser effect on Km, for ?-selinene synthase and afforded a much simpler product spectrum composed largely of monocyclic olefins. Identical alterations in ?-humulene synthase produced similar kinetic effects with a simplified product spectrum of mostly acyclic and monocyclic olefins. Although impaired in product diversity, none of the mutant synthases lost entirely the capacity to generate complex structures. These results confirm the catalytic significance of the DDxxD motifs and imply that they also influence permitted modes of cyclization. Deletion of an N-terminal arginine pair in ?-selinene synthase (an element potentially involved in substrate isomerization) altered kinetics without substantially altering product outcome. Finally, mutation of an active-site tyrosine residue thought to play a role in proton exchange had little influence; however, substitution of a nearby active site aspartate dramatically altered kinetics and product outcome.
Analysis of Terpene and Terpenoid Content in Cannabis Sativa Using Headspace with GC/MSD
Abbey Fausett
Agilent
Terpenes and terpenoids are compounds produced by botanical species to flourish in their environment. The compounds often attract pollinators, repel pests, and assist with adaptation throughout a growth cycle. Chemically, terpenes are comprised of carbon and hydrogen atoms, and are built from isoprene (C5 H8) subunits. Terpenoid describes a larger class of molecules that include oxygen in the chemical structure. Both classes of compounds will be generalized to terpenes for this application note, but they are two distinct classes in the broader scope. Terpenes have an associated fragrance, and have historically been isolated from various botanical sources for a wide range of commercial or therapeutic uses.2 D-limonene is a common component of citrus-scented personal care or disinfecting products, eucalyptol contributes to the minty aoma in many therapeutic products, and linalool is largely responsible for the floral fragrance of lavender-scented products. These terpenes, along with others produced by cannabis plants, are of interest as they are commonly marketed to enhance effects in the population consuming cannabis for medicinal or recreational use.
Analysis of Terpenes in Cannabis sativa L. Using GC/MS: Method Development, Validation, and Application
Elsayed Ibrahim, Mei Wang, Mohamed M Radwan, Mahmoud A Elsohly
Planta Medica 85(5) January 2019
DOI: 10.1055/a-0828-8387
Terpenes are the major components of the essential oils present in various Cannabis sativa L. varieties. These compounds are responsible for the distinctive aromas and flavors. Besides the quantification of the cannabinoids, determination of the terpenes in C. sativa strains could be of importance for the plant selection process. At the University of Mississippi, a GC-MS method has been developed and validated for the quantification of terpenes in cannabis plant material, viz., ?-pinene, ?-pinene, ?-myrcene, limonene, terpinolene, linalool, ?-terpineol, ?-caryophyllene, ?-humulene, and caryophyllene oxide. The method was optimized and fully validated according to AOAC (Association of Official Analytical Chemists) guidelines against reference standards of selected terpenes. Samples were prepared by extraction of the plant material with ethyl acetate containing n-tridecane solution (100 µg/mL) as the internal standard. The concentration-response relationship for all analyzed terpenes using the developed method was linear with r2 values > 0.99. The average recoveries for all terpenes in spiked indoor cultivated samples were between 95.0 – 105.7%, with the exception of terpinolene (67 – 70%). The measured repeatability and intermediate precisions (% relative standard deviation) in all varieties ranged from 0.32 to 8.47%. The limit of detection and limit of quantitation for all targeted terpenes were determined to be 0.25 and 0.75 µg/mL, respectively. The proposed method is highly selective, reliable, and accurate and has been applied to the simultaneous determination of these major terpenes in the C. sativa biomass produced by our facility at the University of Mississippi as well as in confiscated marijuana samples.
Analysis of Terpenes in Cannabis Using Headspace Solid-Phase Microextraction and GC–MS
Katherine K. Stenerson , Michael R. Halpenny
Cannabis Science and Technology February 28, 2018
https://www.cannabissciencetech.com/...ction-and-gcms
Headspace SPME combined with GC–MS for the qualitative and quantitative analysis of terpenes in cannabis offers several advantages compared to other methods. It does not require the use of organic solvents, does not coextract matrix, and provides additional means of peak identification and purity using spectral data. It is also a nondestructive method.
As the legalization of medicinal cannabis continues to sweep across the United States, an urgent need has developed for fast, accurate, and efficient analytical testing. In addition to testing for contaminants and potency, there is also interest in the determination of terpene identity and concentration levels present in different strains of cannabis. Terpenes have been shown to have therapeutic uses for treatment of different medical conditions ranging from cancer and inflammation to anxiety and sleeplessness. It is believed that the combination of terpenes and cannabinoids in cannabis produce a synergistic effect with regards to medical benefits. The traditional testing method for terpenes in plant materials involves a solvent-based extraction followed by gas chromatography (GC) analysis. In this work, headspace solid-phase microextraction (HS-SPME) was used to identify and quantify terpene content in cannabis. The HS-SPME method offered several advantages compared to solvent extraction in that it provided a cleaner analysis, free of interferences from coextracted matrix, and was nondestructive to the sample. A cannabis sample of unknown origin was first analyzed qualitatively by HS-SPME and GC–mass spectrometry (MS). Spectral library matching and retention indices were used to identify 42 terpenes. Quantitative analysis was then performed for several selected terpenes using spiked samples. Method accuracy was >90%, with reproducibility of <5% relative standard deviation (RSD) for analysis of spiked replicates. The HS-SPME results were then compared to an analysis using a conventional solvent extraction method, and the two approaches were found to produce comparable results.
Anticancer and Antioxidant Properties of Terpinolene in Rat Brain Cells.
Aydin, E., Türkez, H., & Taşdemir, Ş.
Archives of Industrial Hygiene and Toxicology, 64(3), 415–424.(2013).
doi:10.2478/10004-1254-64-2013-2365
Terpinolene (TPO) is a natural monoterpene present in essential oils of many aromatic plant species. Although various biological activities of TPO have been demonstrated, its neurotoxicity has never been explored. In this in vitro study we investigated TPO’s antiproliferative and/or cytotoxic properties using the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) test, genotoxic damage potential using the single-cell gel electrophoresis (SCGE), and oxidative effects through total antioxidant capacity (TAC) and total oxidative stress (TOS) in cultured primary rat neurons and N2a neuroblastoma cells. Dose-dependent effects of TPO (at 10 mg L-1, 25 mg L-1, 50 mg L-1, 100 mg L-1, 200 mg L-1, and 400 mg L-1) were tested in both cell types. Significant (P<0.05) decrease in cell proliferation were observed in cultured primary rat neurons starting with the dose of 100 mg L-1 and in N2a neuroblastoma cells starting with 50 mg L-1. TPO was not genotoxic in either cell type. In addition, TPO treatment at 10 mg L-1, 25 mg L-1, and 50 mg L-1 increased TAC in primary rat neurons, but not in N2a cells. However, at concentrations above 50 mg L-1 it increased TOS in both cell types. Our fi ndings clearly demonstrate that TPO is a potent antiproliferative agent for brain tumour cells and may have potential as an anticancer agent, which needs to be further studied.
Antifungal Activity of the Volatiles of High Potency Cannabis sativa L. Against Cryptococcus neoformans
Amira S. Wanas, Mohammed M. Radwan, Zlatko Mehmedic, Melissa Jacob, Iklas A. Khan, and Mahmoud A. Elsohly
Rec. Nat. Prod. 10.2 (2016) 214-220
https://www.researchgate.net/publica...cus_neoformans
The n-hexane extracted volatile fraction of high potency Cannabis sativa L (Cannabaceae). was assessed in vitro for antifungal, antibacterial and antileishmanial activities. The oil exhibited selective albeit modest, antifungal activity against Cryptococcus neoformans with an IC50 value of 33.1 ?g/mL. Biologically-guided fractionation of the volatile fraction resulted in the isolation of three major compounds (1-3) using various chromatographic techniques. The chemical structures of the isolated compounds were identified as ?-humulene (1), ?-caryophyllene (2) and caryophyllene oxide (3) using GC/FID, GC/MS, 1D- and 2D-NMR analyses, respectively. Compound 1 showed potent and selective antifungal activity against Cryptococcus neoformans with IC50 and MIC values of 1.18 ?g/mL and 5.0 ?g/mL respectively. Whereas compound 2 showed weak activity (IC50 19.4 ?g/mL), while compound 3 was inactive against C. neoformans.
Anti-inflammatory Potential of Terpenes Present in Cannabis sativa L.
Eric J. Downer
ACS Chem. Neurosci. XXXX, XXX, XXX?XXX
Doi: 10.1021/acschemneuro.0c00075
Cannabis sativa L. (C. sativa) contains an array of plant-derived (phyto) cannabinoids and terpenes that are predominantly located in the trichome cavity of the plant. Terpenes, aromatic organic hydrocarbons characterized for their role in plant protection/pollination, are gaining attention for their potential as novel therapeutics in many areas of biomedicine. This Viewpoint will explore the exciting recent evidence that terpenes have anti-inflammatory/antioxidant propensity by targeting inflammatory signaling mechanisms relevant to human disease. Given their anti-inflammatory properties, terpenes may contribute to the effects of current cannabinoid-based therapies.
Antitumor effect of 1, 8-cineole against colon cancer.
MURATA, S., SHIRAGAMI, R., KOSUGI, C., TEZUKA, T., YAMAZAKI, M., HIRANO, A., … KODA, K.
Oncology Reports, 30(6), 2647–2652.(2013).
doi:10.3892/or.2013.2763
Several essential oils possess pharmacological effects. Among the various constituents of essential oils, 1, 8-cineole has been shown to possess pharmacological effects such as anti-bacterial and anti-inflammatory effects. The effect of 1, 8-cineole on human colorectal cancer cells, however, has not reported previously. In this study, we have investigated the anti-proliferative effect of 1, 8-cineole on human colon cancer cell lines HCT116 and RKO by WST-8 and BrdU assays. The cytotoxicity of 1, 8-cineole was investigated by LDH activity and TUNEL staining. The mechanism of apoptosis by 1, 8-cineole was determined by western blot analyses. In in vivo study, RKO cells were injected into the SCID mice and the effect of 1, 8-cineole was investigated. Specific induction of apoptosis, not necrosis, was observed in human colon cancer cell lines HCT116 and RKO by 1, 8-cineole. The treatment with 1, 8-cineole was associated with inactivation of survivin and Akt and activation of p38. These molecules induced cleaved PARP and caspase-3, finally causing apoptosis. In xenotransplanted SCID mice, the 1, 8-cineole group showed significantly inhibited tumor progression compared to the control group. These results indicated 1, 8-cineole suppressed human colorectal cancer proliferation by inducing apoptosis. Based on these studies 1, 8-cineole would be an effective strategy to treat colorectal cancer.
NOT CANNABIS SPECIFIC
A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis?
Andrea Hemmerlin, John L. Harwood, Thomas J. Bach
Progress in Lipid Research 51 (2):95–148( 2012)
doi: 10.1016/j.plipres.2011.12.001
When compared to other organisms, plants are atypical with respect to isoprenoid biosynthesis: they utilize two distinct and separately compartmentalized pathways to build up isoprene units. The co-existence of these pathways in the cytosol and in plastids might permit the synthesis of many vital compounds, being essential for a sessile organism. While substrate exchange across membranes has been shown for a variety of plant species, lack of complementation of strong phenotypes, resulting from inactivation of either the cytosolic pathway (growth and development defects) or the plastidial pathway (pigment bleaching), seems to be surprising at first sight. Hundreds of isoprenoids have been analyzed to determine their biosynthetic origins. It can be concluded that in angiosperms, under standard growth conditions, C20-phytyl moieties, C30-triterpenes and C40-carotenoids are made nearly exclusively within compartmentalized pathways, while mixed origins are widespread for other types of isoprenoid-derived molecules. It seems likely that this coexistence is essential for the interaction of plants with their environment. A major purpose of this review is to summarize such observations, especially within an ecological and functional context and with some emphasis on regulation. This latter aspect still requires more work and present conclusions are preliminary, although some general features seem to exist.
Not Cannabis specific
A Review of Terpenes from Marine-Derived Fungi: 2015–2019.
Jiang, M., Wu, Z., Guo, H., Liu, L., & Chen, S.
Marine Drugs, 18(6), 321.(2020).
doi:10.3390/md18060321
Marine-derived fungi are a significant source of pharmacologically active metabolites with interesting structural properties, especially terpenoids with biological and chemical diversity. In the past five years, there has been a tremendous increase in the rate of new terpenoids from marine-derived fungi being discovered. In this updated review, we examine the chemical structures and bioactive properties of new terpenes from marine-derived fungi, and the biodiversity of these fungi from 2015 to 2019. A total of 140 research papers describing 471 new terpenoids of six groups (monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, triterpenes, and meroterpenes) from 133 marine fungal strains belonging to 34 genera were included. Among them, sesquiterpenes, meroterpenes, and diterpenes comprise the largest proportions of terpenes, and the fungi genera of Penicillium, Aspergillus, and Trichoderma are the dominant producers of terpenoids. The majority of the marine-derived fungi are isolated from live marine matter: marine animals and aquatic plants (including mangrove plants and algae). Moreover, many terpenoids display various bioactivities, including cytotoxicity, antibacterial activity, lethal toxicity, anti-inflammatory activity, enzyme inhibitor activity, etc. In our opinion, the chemical diversity and biological activities of these novel terpenoids will provide medical and chemical researchers with a plenty variety of promising lead compounds for the development of marine drugs.
Beta-caryophyllene, a CB2R selective agonist, protects against cognitive impairment caused by neuro-inflammation and not in dementia due to ageing induced by mitochondrial dysfunction
Urja Kanojia, Shrikant Gyaneshwar Chaturbhuj, Runali Sankhe, Maushami Das, Raviteja Surubhotla,Nandakumar Krishnadas, Karthik Gourishetti, Pawan Ganesh Nayak, Anoop Kishore
CNS & Neurological Disorders - Drug Targets
DOI : 10.2174/1871527320666210202121103
Background: Dementia is a neurodegenerative disorder majorly evidenced by cognitive impairment. Although there are many types of dementia, the common underlying etiological factors in all the types are neuro-inflammation or ageing induced apoptosis. ?-caryophyllene, a cannabinoid type-2 receptor agonist has reported to have promising neuroprotective effects in cerebral ischemia and neuro-inflammation.
Objective: In the present study, we evaluated the effects of ?-caryophyllene, against animal models of dementia whose etiology mimicked neuro-inflammation and ageing.
Method: Two doses (50 and 100 mg/kg of body weight) of ?-caryophyllene given orally were tested against AlCl3-induced dementia in male Sprague Dawley (SD) rats using Morris water maze test. Subsequently, the effect of the drug was assessed for episodic memory in female SD rats using novel object recognition task in doxorubicin-induced neuro-inflammation and male SD rats for chemobrain model. Moreover, its effects were evaluated in D-galactose-induced mitochondrial dysfunction leading to dementia.
Results: ?-caryophyllene, at both the doses, showed significant improvement in memory when assessed using parameters like target quadrant entries, escape latency and path efficiency in Morris water maze test for spatial memory. In the doxorubicin-induced chemobrain model, ?-caryophyllene at 100 mg/kg significantly elevated acetylcholinesterase and catalase levels and lowered lipid peroxidation compared to the disease control. In the novel object recognition task, ?-caryophyllene at 100 mg/kg significantly improved recognition index and discrimination index in the treated animals compared to the disease control, with a significant increase in catalase and decrease in lipid peroxidation in both hippocampus and frontal cortex. However, in D-galactose-induced mitochondrial dysfunction model, ?-caryophyllene failed to show positive effects when spatial memory was assessed. It also failed to improve D-galactose induced diminished mitochondrial complex I and II activities.
Beta-Caryophyllene, a CB2-Receptor-Selective Phytocannabinoid, Suppresses Mechanical Allodynia in a Mouse Model of Antiretroviral-Induced Neuropathic Pain.
Aly, E., Khajah, M. A., & Masocha, W.
Molecules, 25(1), 106. (2019).
doi:10.3390/molecules25010106
Neuropathic pain associated with nucleoside reverse transcriptase inhibitors (NRTIs), therapeutic agents for human immunodeficiency virus (HIV), responds poorly to available drugs. Smoked cannabis was reported to relieve HIV-associated neuropathic pain in clinical trials. Some constituents of cannabis (Cannabis sativa) activate cannabinoid type 1 (CB1) and cannabinoid type 2 (CB2) receptors. However, activation of the CB1 receptor is associated with side effects such as psychosis and physical dependence. Therefore, we investigated the effect of ?-caryophyllene (BCP), a CB2-selective phytocannabinoid, in a model of NRTI-induced neuropathic pain. Female BALB/c mice treated with 20 -30 -dideoxycytidine (ddC, zalcitabine), a NRTI, for 5 days developed mechanical allodynia, which was prevented by cotreatment with BCP, minocycline or pentoxifylline. A CB2 receptor antagonist (AM 630), but not a CB1 receptor antagonist (AM 251), antagonized BCP attenuation of established ddC-induced mechanical allodynia. ?-Caryophyllene prevented the ddC-induced increase in cytokine (interleukin 1 beta, tumor necrosis factor alpha and interferon gamma) transcripts in the paw skin and brain, as well as the phosphorylation level of Erk1/2 in the brain. In conclusion, BCP prevents NRTI-induced mechanical allodynia, possibly via reducing the inflammatory response, and attenuates mechanical allodynia through CB2 receptor activation. Therefore, BCP could be useful for prevention and treatment of antiretroviral-induced neuropathic pain.
β-Caryophyllene: A Sesquiterpene with Countless Biological Properties
Fabrizio Francomano, Anna Caruso, Alexia Barbarossa, Alessia Fazio Chiara La Torre, Jessica Ceramella, Rosanna Mallamaci, Carmela Saturnino, Domenico Iacopetta and Maria Stefania Sinicropi
Applied Sciences 2019
DOI: 10.3390/app9245420
β-Caryophyllene (BCP), a natural bicyclic sesquiterpene, is a selective phytocannabinoid agonist of type 2 receptors (CB2-R). It isn’t psychogenic due to the absence of an affinity to cannabinoid receptor type 1 (CB1). Among the various biological activities, BCP exerts anti-inflammatory action via inhibiting the main inflammatory mediators, such as inducible nitric oxide synthase (iNOS), Interleukin 1 beta (IL-1β), Interleukin-6 (IL-6), tumor necrosis factor-alfa (TNF-α), nuclear factor kapp a-light-chain-enhancer of activated B cells (NF-κB), cyclooxygenase 1 (COX-1), cyclooxygenase 2 (COX-2). Peroxisome proliferator-activated receptors alpha (PPAR-α) effects are also mediated by the activation of PPAR-α and PPAR-γ receptors. In detail, many studies, in vitro and in vivo, suggest that the treatment with β-caryophyllene improves the phenotype of animals used to model various inflammatory pathologies, such as nervous system diseases (Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, amyotrophic lateral sclerosis, stroke), atherosclerosis, and tumours (colon, breast, pancreas, lymphoma, melanoma and glioma cancer). Furthermore, pre-clinical data have highlighted that BCP is potentially useful in Streptococcus infections, osteoporosis, steatohepatitis, and exerts anticonvulsant, analgesic, myorelaxing, sedative, and antidepressive effects. BCP is non-toxic in rodents, with a Lethal dose, 50% (LD50) greater than 5000 mg/kg. Nevertheless, it inhibits various cytochrome P450 isoforms (above all, CYP3A4), which metabolise xenobiotics, leading to adverse effects, due to drug levels over therapeutic window. All the reported data have highlighted that both pharmacological and toxicological aspects need to be further investigated with clinical trials
BETA CARYOPHYLLENE - A TERPENE OR A CANNABINOID?
Noel Palmer
https://www.cbxsciences.com/blog/201...-a-cannabinoid
Most people understand that cannabis is responsible for producing cannabinoids; most notably delta-9 THC and CBD. What many people don’t fully understand is that cannabis produces other phytochemicals (chemicals produced by plants) that are therapeutically active. Terpenes are a class of phytochemicals produced by cannabis (and other plants), and in fact scientists believe that terpenes serve as the building blocks for cannabinoids in the cannabis plant. That being said, terpenes in and of themselves are considered to be therapeutically relevant in many ways.
Beta-caryophyllene attenuates short-term recurrent seizure activity and blood-brain-barrier breakdown after pilocarpine-induced status epilepticus in rats
Michele Pereira Mallmann, Fernanda Kulinski Mello, Bruna Neuberger, Karine Gabriela da Costa Sobral, Michele Rechia Fighera, Luiz Fernando Freire Royes, Ana Flávia Furian, Mauro Schneider Oliveira
Brain Res. 2022 Mar 14;1784:147883.
doi: 10.1016/j.brainres.2022.147883.
Background: Status epilepticus (SE) is a neurological life-threatening condition, resulting from the failure of the mechanisms responsible for seizure termination. SE is often pharmacoresistant and associated with significant morbidity and mortality. Hence, ceasing or attenuating SE and its consequences is of fundamental importance. Beta-caryophyllene is a functional CB2 receptor agonist and exhibit a good safety profile. Besides, it displays beneficial effects in several experimental conditions, including neuroprotective activity. In the present study we aimed to investigate the effects of beta-caryophyllene on pilocarpine-induced SE.
Methods: Wistar rats were submitted to pilocarpine-induced SE and monitored for 24 h by video and EEG for short-term recurrence of seizure activity (i.e. seizures occurring within 24 h after termination of SE). Rats received beta-caryophyllene (100 mg/kg, ip) at 1, 8- and 16-hours after SE. Twenty-four hours after SE we evaluated sensorimotor response, neuronal damage (fluoro jade C staining) and serum albumin infiltration into brain parenchyma.
Beta-caryophyllene is a dietary cannabinoid
Jurg Gertsch, Marco Leonti, Stefan Raduner, Ildiko Racz, Jian-Zhong Chen, Xiang-Qun Xie , Karl-Heinz Altmann, Meliha Karsak, and Andreas Zimmer
PNAS July 1, 2008 vol. 105 no. 26 9099–9104 PH
DOI: 10.1073/pnas.0803601105
The psychoactive cannabinoids from Cannabis sativa L. and the arachidonic acid-derived endocannabinoids are nonselective natural ligands for cannabinoid receptor type 1 (CB1) and CB2 receptors. Although the CB1 receptor is responsible for the psychomodulatory effects, activation of the CB2 receptor is a potential therapeutic strategy for the treatment of inflammation, pain, atherosclerosis, and osteoporosis. Here, we report that the widespread plant volatile (E)--caryophyllene [(E)-BCP] selectively binds to the CB2 receptor (Ki 155 4 nM) and that it is a functional CB2 agonist. Intriguingly, (E)-BCP is a common constituent of the essential oils of numerous spice and food plants and a major component in Cannabis. Molecular docking simulations have identified a putative binding site of (E)-BCP in the CB2 receptor, showing ligand stacking interactions with residues F117 and W258. Upon binding to the CB2 receptor, (E)-BCP inhibits adenylate cylcase, leads to intracellular calcium transients and weakly activates the mitogen-activated kinases Erk1/2 and p38 in primary human monocytes. (E)-BCP (500 nM) inhibits lipopolysaccharide (LPS)-induced proinflammatory cytokine expression in peripheral blood and attenuates LPS-stimulated Erk1/2 and JNK1/2 phosphorylation in monocytes. Furthermore, peroral (E)-BCP at 5 mg/kg strongly reduces the carrageenan-induced inflammatory response in wild-type mice but not in mice lacking CB2 receptors, providing evidence that this natural product exerts cannabimimetic effects in vivo. These results identify (E)-BCP as a functional nonpsychoactive CB2 receptor ligand in foodstuff and as a macrocyclic antiinflammatory cannabinoid in Cannabis.
Busting the THC Myth: When it Comes to the Best User Experience, Terpenes Reign Supreme
Mark Lange
Cannabis Business Journal March 3 2022
https://cannabisindustryjournal.com/column/busting-the-thc-myth-when-it-comes-to-the-best-user-experience-terpenes-reign-supreme/
We have just begun to scratch the surface of the potential of terpenes in cannabis. With the right alignment across the industry and a stronger focus on genetics in breeding, we will see the rise of completely unique cannabis varieties.
The scent of pine from your Christmas tree. The fragrance of a ripe summer peach at the farmer’s market. The whiff of eucalyptus and lavender that greets you when you enter a spa.
Aroma is a keystone in how we experience the world. In any given environment, aroma can help shape your mood, solidify memories and instantly transport you to another place or time.
I have focused my career on studying the fascinating compounds that are often behind these powerful aromas: terpenes. They form the largest class of natural products (compounds produced by living organisms), found in nearly all living beings. There are around 50,000 currently known terpenes in nature — with potentially thousands yet to be discovered.
Terpene-rich plants you might be most familiar with are lavender, mint, oranges (in the peel), and yes, cannabis. In recent years, terpenes have rightfully become a central discussion in the recreational cannabis world. This is because terpenes — not THC level, not “Indica-Sativa” classification — are a key determinant of cannabis’s effect, both psychoactive and non-psychoactive. But the current lack of prioritization and understanding of the crucial role terpenes play may put the collective quality of U.S. cannabis at risk.
At this crucial inflection point for legal cannabis, on its path to becoming a $70 billion dollar global industry by 2028, we need to ensure that everyone across the cannabis space, from breeders to testers, growers and consumers, understands which traits to prioritize for a cannabis world brimming with diversity and predictable effects.
What the cannabis industry has to lose
What do we lose if the cannabis industry continues to scale without a clear understanding of the compounds that define the uniqueness of each variety?
There is a ripple effect across the ecosystem. For cannabis testing labs, focusing on only twenty of the most dominant terpenes means we are missing out on tapping into potentially over a hundred of less common terpenes in cannabis. For the cannabis consumer, lack of understanding on the breeding and testing side may make it difficult to find cannabis that delivers on its promised effect time and time again. And, most detrimentally for breeders, not understanding the direct correlation between genetics and the formation of terpenes means we will have increasingly fewer terpene profiles and combinations to work with, especially when the industry-dominant focus has been on cannabinoid potency.
Let’s explore some misconceptions related to potency. In recent years, many breeders have prioritized high THC levels over genetic diversity. Consumers often associate high THC levels and that telltale strong “skunky” aroma with a strain’s quality and effect, when in reality, these are poor indicators of potency. (In fact, recent research indicates that this specific cannabis aroma is caused by a family of sulfur compounds.) Terpene profiling is a much more accurate way to determine a variety’s given effect. In focusing too much on increasing THC, breeders miss out on the true potency powerhouse: tapping into the terpene diversity that’s out there. Terpenes are responsible for giving flowers (including cannabis), fruits and spices their distinctive flavors and aromas. Common terpenes include limonene, linalool, pinene and myrcene.
Cannabinoid Receptors Are Absent in Insects
JOHN MCPARTLAND, VINCENZO DI MARZO, LUCIANO DE PETROCELLIS, LISON MERCER, AND MICHELLE GLASS
THE JOURNAL OF COMPARATIVE NEUROLOGY 436:423–429 (2001)
doi: 10.1002/cne.1078
The endocannabinoid system exerts an important neuromodulatory role in mammals. Knockout mice lacking cannabinoid (CB) receptors exhibit significant morbidity. The endocannabinoid system also appears to be phylogenetically ancient—it occurs in mammals, birds, amphibians, fish, sea urchins, leeches, mussels, and even the most primitive animal with a nerve network, the Hydra. The presence of CB receptors, however, has not been examined in terrestrial invertebrates (or any member of the Ecdysozoa). Surprisingly, we found no specific binding of the synthetic CB ligands [3
H]CP55,940 and [3 H]SR141716A in a panel of insects: Apis mellifera, Drosophila melanogaster, Gerris marginatus, Spodoptera frugiperda, and Zophobas atratus. A lack of functional CB receptors was confirmed by the inability of tetrahydrocannabinol (THC) and HU210 to activate G-proteins in insect tissues, utilizing a guanosine-59-O-(3-[35]thio)-triphosphate (GTPgS) assay. No orthologs of human CB receptors were located in the Drosophila genome, nor did we find orthologs of fatty acid amide hydrolase. This loss of CB receptors appears to be unique in the field of comparative
neurobiology. No other known mammalian neuroreceptor is understood to be missing in insects. We hypothesized that CB receptors were lost in insects because of a dearth of ligands; endogenous CB ligands are metabolites of arachidonic acid, and insects produce little or no arachidonic acid or endocannabinoid ligands, such as anandamide.
Cannabinoids and terpenes as chemotaxonomic markers in cannabis.
Elzinga S, Fischedick J, Podkolinski R, Raber JC (2015)
Nat Prod Chem Res 3: 2
DOI: 10.4172/2329-6836.1000181
https://www.researchgate.net/profile...8c85c016aa.pdf
In this paper, we present principal component analysis (PCA) results from a dataset containing 494 cannabis
flower samples and 170 concentrate samples analyzed for 31 compounds. A continuum of chemical composition
amongst cannabis strains was found instead of distinct chemotypes. Our data shows that some strains are much
more reproducible in chemical composition than others. Strains labeled as indica were compared with those labeled
as sativa and no evidence was found that these two cultivars are distinctly different chemotypes. PCA of “OG” and
“Kush” type strains found that “OG” strains have relatively higher levels of ?-terpineol, fenchol, limonene, camphene, terpinolene and linalool where “Kush” samples are characterized mainly by the compounds trans-ocimene, guaiol, ?-eudesmol,myrcene and ?-pinene. The composition of concentrates and flowers were compared as well. Although the absolute concentration of compounds in concentrates is much higher, the relative composition of compounds between flowers and concentrates is similar.
Cannabinoid synthases and osmoprotective metabolites accumulate in the exudates of Cannabis sativa L. glandular trichomes.
Pawe Rodziewicz, Stefan Loroch, Lukasz Marczak, Oliver Kayser
April 2019 Plant Science 284
doi: 10.1016/j.plantsci.2019.04.008
Cannabinoids are terpenophenolic compounds produced by Cannabis sativa L., which accumulate in storage cavities of glandular trichomes as a part of the exudates. We investigated if tetrahydrocannabinolic acid synthase and cannabidiolic acid synthase, which are involved in the last step of cannabinoid biosynthesis, are also secreted into Cannabis trichome exudates. The exudates were collected by microsuction from storage cavities of Cannabis glandular trichomes and were subjected for proteomic and metabolomic analyses. The catalytic activity of the exudates was documented by cannabigerolic acid biotransformation studies under hydrophobic conditions. Electrophoretic separations revealed protein bands at ˜65 kDa, which were further identified as tetrahydrocannabinolic acid synthase and cannabidiolic acid synthase. The accumulation of the enzymes in trichome exudates increased substantially during the flowering period in the drug-type Cannabis plants. The content of cannabinoids increased significantly after incubating hexane-diluted trichome exudates with cannabigerolic acid. In this study, we showed that Cannabis glandular trichomes secrete and accumulate cannabinoid synthases in storage cavities, and the enzymes able to convert cannabigerolic acid under hydrophobic trichome-mimicking conditions. Metabolite profiling of the exudates revealed compounds with hydrophilic, osmoprotective and amphiphilic properties, which may play a role in providing a necessary aqueous microenvironment, which enables enzyme solubility and biocatalysis under hydrophobic conditions of glandular trichomes.
Cannabis chemovar classification: terpenes hyper-classes and targeted genetic markers for accurate discrimination of flavours and effects
Philippe Henry 2017
Doi:10.7287/peerj.preprints.3307v1
The classification of Cannabis varieties has been increasingly discussed in the past years, particularly in the wake of emerging legal markets, with implications for intellectual property development, marketing and improvement of the scientific understanding of this contentious plant. While the concept of chemovars has been proposed and has gained popularity of late, the lack of guidance in introducing this concept and the fact that chemovars are based on indirectly assessed traits with a heritable basis has likely impeded the implementation of the concept to a broader audience. Here I propose a simplified version of terpene hyper-classes based on three dominant terpenes that is shown to outperformed the classic indica-sativa-hybrid scheme of classification as well as a recently proposed terpene super-class scheme. This information was used to identify the most informative genetic markers for chemovar classification based on the terpene hyper-classes. I demonstrate the ability of clearly clustering accessions based on their dominant terpene and propose to extent this approach as a benchmark for chemovar classification in lieu of previously proposed models.
Cannabis Essential Oil: A Preliminary Study for the Evaluation of the Brain Effects
Nadia Gulluni , Tania Re , Idalba Loiacono, Giovanni Lanzo, Luigi Gori, Claudio Macchi, Francesco Epifani, Nicola Bragazzi , and Fabio Firenzuoli
Hindawi
Evidence-Based Complementary and Alternative Medicine Volume 2018, Article ID 1709182, 1-11 pages
doi: 10.1155/2018/1709182
We examined the effects of essential oil from legal (THC <0.2% w/v) hemp variety on the nervous system in 5 healthy volunteers. GC/EIMS and GC/FID analysis of the EO showed that the main components were myrcene and Beta-caryophyllene.The experiment consisted of measuring autonomic nervous system (ANS) parameters; evaluations of the mood state; and electroencephalography (EEG) recording before treatment, during treatment, and after hemp inhalation periods as compared with control conditions. The results revealed decreased diastolic blood pressure, increased heart rate, and significant increased skin temperature. The subjects described themselves as more energetic, relaxed, and calm. The analysis EEG showed a significant increase in the mean frequency of alpha (8–13Hz) and significant decreased mean frequency and relative power of beta 2 (18,5–30Hz) waves.Moreover, an increased power, relative power, and amplitude of theta (4–8Hz) and alpha brain waves activities and an increment in the delta wave (0,5–4Hz) power and relative power was recorded in the posterior region of the brain. These results suggest that the brain wave activity and ANS are affected by the inhalation of the EO of Cannabis sativa suggesting a neuromodular activity in cases of stress, depression, and anxiety.
Cannabis labelling is associated with genetic variation in terpene synthase genes
Sophie Watts, Michel McElroy, Zoë Migicovsky, Hugo Maassen, Robin van Velzen, and Sean Myles
Nature Plants | VOL 1330 7 | Octobe r 2021 | 1330–1334
DOI: 10.1038/s41477-021-01003-y
https://www.nature.com/articles/s41477-021-01003-y.pdf
Analysis of over 100 Cannabis samples quantified for terpene and cannabinoid content and genotyped for over 100,000 single nucleotide polymorphisms indicated that Sativa- and Indica-labelled samples were genetically indistinct on a genome-wide scale. Instead, we found that Cannabis labeling was associated with variation in a small number of terpenes whose concentrations are controlled by genetic variation at tandem arrays of terpene synthase genes
Cannabis Pharmacology:The Usual Suspects and a Few Promising Leads
Ethan Russo
Poster
https://ethanrusso.org/download/cann...omising-leads/
Outline of an Ideal Cannabis Classification Scheme
Combines shape, content and purpose
Basic class based on primary cannabinoid (e.g. Type I for THC)
Plant morphology (e.g., broad-leaf, compact vs. tall, spindly)
Specific cannabinoid content
Specific terpenoid content
Scent
Taste (when vaporized)
Uses/Effects (patient-oriented)
Cannabis Sativa L.: a comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization.
Micalizzi G, Vento F, Alibrando F, Donnarumma D, Dugo P, Mondello L.
J Chromatogr A. 2020 Dec 30;1637:461864.
doi: 10.1016/j.chroma.2020.461864.
Undoubtedly, the enormous interest about cannabis cultivation mainly derives from the well-known pharmacological properties of cannabinoids and terpenes biosynthesized by the plants. ...Lastly, GC GC techniques are also reported for accurate identification and quantificatification of terpenes in complex cannabis matrices.
Cannabis sativa Terpenes are Cannabimimetic and Provide Support for the Entourage Effect Hypothesis
Justin E. LaVigne, Ryan Heckse, Attila Keresztes, John M. Streiche
Nature Scientific Reports
DOI: 10.1101/2020.10.22.350868
https://www.nature.com/articles/s41598-021-87740-8.pdf
Limited evidence has suggested that terpenes found in Cannabis sativa are analgesic, and could produce an “entourage efect” whereby they modulate cannabinoids to result in improved outcomes. However this hypothesis is controversial, with limited evidence. We thus investigated Cannabis sativa terpenes alone and with the cannabinoid agonist WIN55,212 using in vitro and in vivo approaches. We found that the terpenes α-humulene, geraniol, linalool, and β-pinene produced cannabinoid tetrad behaviors in mice, suggesting cannabimimetic activity. Some behaviors could be blocked by cannabinoid or adenosine receptor antagonists, suggesting a mixed mechanism of action. These behavioral efects were selectively additive with WIN55,212, suggesting terpenes can boost cannabinoid activity. In vitro experiments showed that all terpenes activated the CB1R, while some activated other targets. Our fndings suggest that these Cannabis terpenes are multifunctional cannabimimetic ligands that provide conceptual support for the entourage efect hypothesis and could be used to enhance the therapeutic properties of cannabinoids.
Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity
Justin E. LaVigne, Ryan Heckse, Attila Keresztes, John M. Streiche
Nature Scientific Reports
DOI: 10.1038/s41598-021-87740-8
Limited evidence has suggested that terpenes found in Cannabis sativa are analgesic, and could produce an “entourage effect” whereby they modulate cannabinoids to result in improved outcomes. However this hypothesis is controversial, with limited evidence. We thus investigated Cannabis sativa terpenes alone and with the cannabinoid agonist WIN55,212 using in vitro and in vivo approaches. We found that the terpenes α-humulene, geraniol, linalool, and β-pinene produced cannabinoid tetrad behaviors in mice, suggesting cannabimimetic activity. Some behaviors could be blocked by cannabinoid or adenosine receptor antagonists, suggesting a mixed mechanism of action. These behavioral effects were selectively additive with WIN55,212, suggesting terpenes can boost cannabinoid activity. In vitro experiments showed that all terpenes activated the CB1R, while some activated other targets. Our findings suggest that these Cannabis terpenes are multifunctional cannabimimetic ligands that provide conceptual support for the entourage effect hypothesis and could be used to enhance the therapeutic properties of cannabinoids.
Challenges and Opportunities for the Analysis of Terpenes in Cannabis
Terry Rodney, Patrisha Pham-Bugayong, Lakshmi C. Kasi Viswanath, Ghalib A Bello, Gerard Dumancas, Bryan John Subong
https://www.researchgate.net/publica...es_in_Cannabis
Cannabis is a complex plant with over 400 chemical entities of which more than 60 of them are cannabinoids. While cannabinoids are the primary psychoactive and medicinal components of cannabis, volatile terpenes contribute to the many significant fragrance attributes that ultimately influence consumer preference for cannabis. There are over 120 different terpene compounds that have been identified in the Cannabis sativa plant alone. Analysis of terpenes in cannabis is extremely important because they contribute to its potency and sensory perceptions. Current methods of quantifying terpenes in cannabis involve the use of chromatographic techniques. However, such techniques require sample preparation, are time-consuming, and the instrument involved can be expensive and requires a skilled operator. The use of Fourier Transform Infrared spectroscopy and chemometrics offer a fast, non-destructive, and affordable means of analyzing terpenes in cannabis. This manuscript will discuss challenges in cannabis terpene analysis using the aforementioned methods including method fragmentation and method multiplicity as well as issues related to its legal use. In general, the cannabis testing industry is poised for a breakthrough in the field of analytical science given the recent laws legalizing its medicinal use as well as advances in the field of spectroscopic miniaturization.
Characterization of cannabis cultivars based on terpene synthase gene profiles (Patent) WO 2022/006019 A1
Jan 2022
Front RangeBiosciences Inc.
ChristopherStephen Pauli, Anthony Torres, Reginald Gaudino, Keith Allen, Thomas Blank, Kymron deCesare https://www.researchgate.net/publica...terization_of_ cannabis_cultivars_based_on_te rpene_synthase_gene_profiles
Characterizing the smell of marijuana by odor impact of volatile compounds: an application of simultaneous chemical and sensory analysis.
Rice Somchai, Koziel JA (2015)
PloS One Published: December 10, 2015
doi:10.1371/journal.pone.0144160
https://journals.plos.org/plosone/ar...type=printable
Recent US legislation permitting recreational use of marijuana in certain states brings the use of marijuana odor as probable cause for search and seizure to the forefront of forensic science, once again. This study showed the use of solid-phase microextraction with multidimensional gas chromatography—mass spectrometry and simultaneous human olfaction to characterize the total aroma of marijuana. The application of odor activity analysis offers an explanation as to why high volatile chemical concentration does not equate to most potent odor impact of a certain compound. This suggests that more attention should be focused on highly odorous compounds typically present in low concentrations, such as nonanal, decanol, o-cymene, benzaldehyde, which have more potent odor impact than previously reported marijuana headspace volatiles.
Chemical Characterization and Evaluation of the Antibacterial Activity of Essential Oils from Fibre-Type Cannabis sativa L. (Hemp).
Iseppi, R., Brighenti, V., Licata, M., Lambertini, A., Sabia, C., Messi, P., … Benvenuti, S.
Molecules, 24(12), 2302. (2019).
doi:10.3390/molecules24122302
Volatile terpenes represent the largest group of Cannabis sativa L. components and they are responsible for its aromatic properties. Even if many studies on C. sativa have been focused on cannabinoids, which are terpenophenolics, little research has been carried out on its volatile terpenic compounds. In the light of all the above, the present work was aimed at the chemical characterization of seventeen essential oils from different fibre-type varieties of C. sativa (industrial hemp or hemp) by means of GC-MS and GC-FID techniques. In total, 71 compounds were identified, and the semi-quantitative analysis revealed that ?- and ?-pinene, ?-myrcene and ?-caryophyllene are the major components in all the essential oils analysed. In addition, a GC-MS method was developed here for the first time, and it was applied to quantify cannabinoids in the essential oils. The antibacterial activity of hemp essential oils against some pathogenic and spoilage microorganisms isolated from food and food processing environment was also determined. The inhibitory effects of the essential oils were evaluated by both the agar well diffusion assay and the minimum inhibitory concentration (MIC) evaluation. By using the agar diffusion method and considering the zone of inhibition, it was possible to preliminarily verify the inhibitory activity on most of the examined strains. The results showed a good antibacterial activity of six hemp essential oils against the Gram-positive bacteria, thus suggesting that hemp essential oil can inhibit or reduce bacterial proliferation and it can be a valid support to reduce microorganism contamination, especially in the food processing field
Chemical Composition of Volatile Oils of Fresh and Air-Dried Buds of Cannabis chemovars, Their Insecticidal and Repellent Activities
Amira S. Wanas, Mohamed M. Radwan, Suman Chandra, Hemant Lata, Zlatko Mehmedic, Abbas Ali, KHC Baser, Betul Demirci, and Mahmoud A. ElSohly
Natural Product Communications Volume 15(5): 1–7
DOI: 10.1177/1934578X20926729
The volatile oils of fresh and air-dried buds of 3 different varieties of Cannabis, namely, high cannabidiol (CBD) chemotype, intermediate CBD/tetrahydrocannabinol (THC) chemotype, and high THC chemotype were prepared by hydrodistillation. Gas chromatography analysis of the volatile oils resulted in the identification of 71 compounds, of which 33 were monoterpenes and 38 were sesquiterpenes. The volatile oil obtained from the THC chemotype showed an increase in the ratio of the sesquiterpenes to monoterpenes content. The content of terpinolene was dramatically decreased upon drying of THC chemotype. Moderate increase in ?-caryophyllene and caryophyllene oxide was observed. However, there was no detectable change in the percentage of monoterpenes and sesquiterpenes content in both the intermediate type and CBD chemotype upon drying. The insecticidal activity of the volatile oils was evaluated. The oil obtained from the fresh and dried high CBD cannabis showed good biting deterrent activity at 10 ug/cm2 compared with N,N-diethyl-meta-toluamide at 4.78 µg/cm2 , and good larvicidal activity
Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study
Gokhan Zengin, Luigi Menghini, Antonella Di Sotto, Romina Mancinelli,
Francesca Sisto, Simone Carradori, Stefania Cesa, Caterina Fraschetti,
Antonello Filippi, Letizia Angiolella, Marcello Locatelli, Luisa Mannina,
Cinzia Ingallina, Valentina Puca, Marianna D’Antonio and Rossella Grande
Molecules 2018, 23, 3266;
doi:10.3390/molecules23123266
Due to renewed interest in the cultivation and production of Italian Cannabis sativa L., we proposed a multi-methodological approach to explore chemically and biologically both the essential oil and the aromatic water of this plant. We reported the chemical composition in terms of cannabinoid content, volatile component, phenolic and flavonoid pattern, and color characteristics. Then, we demonstrated the ethnopharmacological relevance of this plant cultivated in Italy as a source of antioxidant compounds toward a large panel of enzymes (pancreatic lipase, ?-amylase, ?-glucosidase, and cholinesterases) and selected clinically relevant, multidrug-sensible, and multidrug-resistant microbial strains (Staphylococcus aureus, Helicobacter pylori, Candida, and Malassezia spp.), evaluating the cytotoxic effects against normal and malignant cell lines. Preliminary in vivo cytotoxicity was also performed on Galleria mellonella larvae. The results corroborate the use of this natural product as a rich source of important biologically active molecules with particular emphasis on the role exerted by naringenin, one of the most important secondary metabolites.
Classification of Common California Cannabis Cultivars via Secondary Metabolite Characterization
Mark A. Lewis
https://www.medicinalgenomics.com/wp...annMed2016.pdf
POSTER
Several Years to Achieve Several Goals:
1. Validated assays & analysis to communicate data
2. Collect data & valid survey of ‘chemoscape’
3. Analysis 1: Class cultivars based on chemotype
4. Analysis 2: Observe intra-genotypic variability
5. Analysis 3: Asexual vs. hybrid seed comparison
Not Cannabis specific
Comparative Study of Steam Distillation and Soxhlet for the Extraction of Botanical Oils
Chibuzor Onyinye Okonkwo and Obioma Christopher Ohaeri
Asian Journal of Biological Sciences Jan 2020
DOI: 10.3923/ajbs.2020.62.69
https://www.researchgate.net/publica...Botanical_Oils
Background and Objective: Steam distillation and soxhlet extraction are among the most commonly used methods in the laboratory
for the extraction of biological compounds. This study was aimed at comparing the chemical composition of insecticidal oils extracted
via these two methods.
Materials and Methods: Oils were extracted from both plants via steam distillation and soxhlet extraction
methods. Extracted oils were then subjected to Gas Chromatography-Mass Spectrometry (GC-MS) analysis to investigate the chemical
components of oils.
Results: The steam distilled oils from both plants contained fewer compounds relative to the soxhlet extracted oils
which contained a wider array of chemical compounds including; phenols, acyclic olefins, esters, ketones, carboxylic acids and alcohols.
Conclusion: Steam distillation alone may not be sufficient to extract most biological compounds present in plant oils.
Not Cannabis specific
Combinatorial Evolution of a Terpene Synthase Gene Cluster Explains Terpene Variations in Oryza.
Chen, H., Köllner, T. G., Li, G., Wei, G., Chen, X., Zeng, D., … Chen, F.
Plant Physiology, pp.00948.2019.
doi:10.1104/pp.19.00948
Terpenes are specialized metabolites ubiquitously produced by plants via the action of terpene synthases (TPSs). There are enormous variations in the types and amounts of terpenes produced by individual species. To understand the mechanisms responsible for such vast diversity, here we investigated the origin and evolution of a cluster of tandemly arrayed TPS genes in Oryza. In the Oryza species analyzed, TPS genes occur as a three-TPS cluster, a two-TPS cluster, and a single TPS gene in five, one, and one species, respectively. Phylogenetic analysis revealed the origins of the two-TPS and three-TPS clusters and the role of species-specific losses of TPS genes. Within the three-TPS clusters, one orthologous group exhibited conserved catalytic activities. The other two groups, both of which contained pseudogenes and/or nonfunctional genes, exhibited distinct profiles of terpene products. Sequence and structural analyses combined with functional validation identified several amino acids in the active site that are critical for catalytic activity divergence of the three orthologous groups. In the five Oryza species containing the three-TPS cluster, their functional TPS genes showed both conserved and species-specific expression patterns in insect-damaged and untreated plants. Emission patterns of volatile terpenes from each species were largely consistent with the expression of their respective TPS genes and the catalytic activities of the encoded enzymes. This study indicates the importance of combinatorial evolution of TPS genes in determining terpene variations among individual species, which includes gene duplication, retention/loss/degradation of duplicated genes, varying selection pressure, retention/divergence in catalytic activities, and divergence in expression regulation.
Not Cannabis Specific
Converting S-limonene synthase to pinene or phellandrene synthases reveals the plasticity of the active site.
Xu, J., Ai, Y., Wang, J., Xu, J., Zhang, Y., & Yang, D.
Phytochemistry, 137, 34–41. (2017).
doi:10.1016/j.phytochem.2017.02.017
S-limonene synthase is a model monoterpene synthase that cyclizes geranyl pyrophosphate (GPP) to form S-limonene. It is a relatively specific enzyme as the majority of its products are composed of limonene. In this study, we converted it to pinene or phellandrene synthases after introducing N345A/ L423A/S454A or N345I mutations. Further studies on N345 suggest the polarity of this residue plays a critical role in limonene production by stabilizing the terpinyl cation intermediate. If it is mutated to a non-polar residue, further cyclization or hydride shifts occurs so the carbocation migrates towards the pyrophosphate, leading to the production of pinene or phellandrene. On the other hand, mutant enzymes that still possess a polar residue at this position produce limonene as the major product. N345 is not the only polar residue that may stabilize the terpinyl cation because it is not strictly conserved among limonene synthases across species and there are also several other polar residues in this area. These residues could form a “polar pocket” that may collectively play this stabilizing role. Our study provides important insights into the catalytic mechanism of limonene synthases. Furthermore, it also has wider implications on the evolution of terpene synthases
Not Cannabis Specific
Crystal Structure of Pentalenene Synthase: Mechanistic Insights on Terpenoid Cyclization Reactions in Biology.
Charles A. Lesburg, Guangzhi Zhai, David E. Cane, David W. Christianson
Science, 277(5333), 1820–1824.(1997).
doi:10.1126/science.277.5333.1820
The crystal structure of pentalenene synthase at 2.6 angstrom resolution reveals critical active site features responsible for the cyclization of farnesyl diphosphate into the tricyclic hydrocarbon pentalenene. Metal-triggered substrate ionization initiates catalysis, and the a-barrel active site serves as a template to channel and stabilize the conformations of reactive carbocation intermediates through a complex cyclization cascade. The core active site structure of the enzyme may be preserved among the greater family of terpenoid synthases, possibly implying divergence from a common ancestral synthase to satisfy biological requirements for increasingly diverse natural products.
Not Cannabis specific
Designed divergent evolution of enzyme function
Yasuo Yoshikuni, Thomas E Ferrin, Jay D Keasling
Nature 440(7087):1078-82 May 2006
DOI: 10.1038/nature04607
https://www.researchgate.net/publica...nzyme_function
It is generally believed that proteins with promiscuous functions divergently evolved to acquire higher specificity and activity, and that this process was highly dependent on the ability of proteins to alter their functions with a small number of amino acid substitutions (plasticity). The application of this theory of divergent molecular evolution to promiscuous enzymes may allow us to design enzymes with more specificity and higher activity. Many structural and biochemical analyses have identified the active or binding site residues important for functional plasticity (plasticity residues). To understand how these residues contribute to molecular evolution, and thereby formulate a design methodology, plasticity residues were probed in the active site of the promiscuous sesquiterpene synthase gamma-humulene synthase. Identified plasticity residues were systematically recombined based on a mathematical model in order to construct novel terpene synthases, each catalysing the synthesis of one or a few very different sesquiterpenes. Here we present the construction of seven specific and active synthases that use different reaction pathways to produce the specific and very different products. Creation of these enzymes demonstrates the feasibility of exploiting the underlying evolvability of this scaffold, and provides evidence that rational approaches based on these ideas are useful for enzyme design.
Differentiation of marijuana headspace volatiles from other plants and hemp products using capillary microextraction of volatiles (CMV) coupled to gas-chromatography–mass spectrometry (GC–MS).
Wiebelhaus N, Kreitals NM, Almirall JR (2016)
Forensic Chem 2: 1–8
doi: 10.1016/j.forc.2016.08.004
The ability to rapidly detect illicit drugs, such as marijuana, is critical to policing legislation across the country. However, it is often difficult to distinguish or identify small quantities of drugs in large spaces without the aid of trained canines. A new device, the capillary microextractor of volatiles (CMV), has the potential to provide rapid detection due to its ability to collect and preconcentrate volatile organic compounds (VOCs) directly from air within minutes. Analysis of the captured compounds can then be performed using a gas chromatography–mass spectrometer (GC–MS). This study focuses on the detection of marijuana volatiles using the CMV as a sampling and preconcentration device given the hypothesis that marijuana will have a distinct chemical profile, or collection of VOCs, that distinguishes it from related plants and other products that could emit similar compounds. Volatile compounds from the headspace of marijuana, related plants, and hemp products were extracted using the CMV and analyzed with GC–MS. The compounds identified and the chemical profiles of each sample were then compared to the volatiles found in the headspace of authentic marijuana samples. The findings presented here suggest that marijuana plants emit volatiles that are readily distinguished from the other samples tested in this study. The distinguishing compounds included a-santalene, valencene, and b-bisabolene. In some cases, THC and cannabinol were also present in the headspace of marijuana. Although these findings support the hypothesis that marijuana has a distinct chemical VOC signature, further work to create a larger database of potential plants and materials is recommended prior to routine use of the CMV coupled to a GC–MS in forensic casework.
Not Cannabis specific
Effect of Soil Nutrient on Production and Diversity of Volatile Terpenoids from Plants
E Ormeño, and C Fernandez
Curr Bioact Compd. 2012 Jan; 8(1): 71–79.
DOI: 10.2174/157340712799828188
Terpenoid production (emission and storage) within foliage plays direct and indirect defensive and protective functions for the plant, mediates complex trophic relationships and controls the oxidation capacity of the atmosphere. Both biotic and abiotic conditions alter terpenoid production, with herbivory, light and temperature effects being reasonably well understood. In this manuscript, the state of the science about nutrient effect on terpenoid production is reviewed. The focus is on isoprene emissions and mono- and sesquiterpenoid
Effect of the Distillation Time on the Chemical Composition, Antioxidant Potential and Antimicrobial Activity of Essential Oils from Different Cannabis sativa L. Cultivars
Sara Palmieri, Francesca Maggio, Marika Pellegrini, Antonella Ricci, Annalisa Serio, Antonello Paparella and Claudio Lo Sterzo
Molecules 2021, 26, 4770.
GOI: 10.3390/ molecules26164770
https://www.ncbi.nlm.nih.gov/pmc/art...s-26-04770.pdf
Within the unavoidable variability of various origins in the characteristics of essential oils, the aim of this study was to evaluate the effect of the distillation time on the chemical composition and biological activity of Cannabis sativa essential oils (EOs). The dry inflorescences came from Carmagnola, Kompolti, Futura 75, Gran Sasso Kush and Carmagnola Lemon varieties from Abruzzo region (Central Italy), the last two being new cultivar here described for the first time. EOs were collected at 2 h and 4 h of distillation; GC/MS technique was applied to characterize their volatile fraction. The EOs were evaluated for total polyphenol content (TPC), antioxidant capacity (AOC) and antimicrobial activity against food-borne pathogens and spoilage bacteria. The time of distillation particularly influenced EOs chemical composition, extracting more or less terpenic components, but generally enriching with minor sesquiterpenes and cannabidiol. A logical response in ratio of time was observed for antioxidant potential, being the essential oils at 4 h of distillation more active than those distilled for 2 h, and particularly Futura 75. Conversely, except for Futura 75, the effect of time on the antimicrobial activity was variable and requires further investigations; nevertheless, the inhibitory activity of all EOs against Pseudomonas fluorescens P34 was an interesting result.
Endophytic fungi harbored in Cannabis sativa L.: diversity and potential as biocontrol agents against host plant-specific phytopathogens
Parijat Kusari & Souvik Kusari & Michael Spiteller & Oliver Kayser
Fungal Diversity (2013) 60:137–151
DOI 10.1007/s13225-012-0216-3
The objective of the present work was isolation, phylogenetic characterization, and assessment of biocontrol potential of endophytic fungi harbored in various tissues (leaves, twigs, and apical and lateral buds) of the medicinal plant, Cannabis sativa L. A total of 30 different fungal endophytes were isolated from all the plant tissues which were authenticated by molecular identification based on rDNA ITS sequence analysis (ITS1, 5.8S and ITS2 regions). The Menhinick’s index revealed that the buds were immensely rich in fungal species, and Camargo’s index showed the highest tissue-specific fungal dominance for the twigs. The most dominant species was Penicillium copticola that could be isolated from the twigs, leaves, and apical and lateral buds. A detailed calculation of Fisher’s log series index, Shannon diversity index, Simpson’s index, Simpson’s diversity index, and Margalef’s richness revealed moderate overall biodiversity of C. sativa endophytes distributed among its tissues. The fungal endophytes were challenged by two host phytopathogens, Botrytis cinerea and Trichothecium roseum, devising a dual culture antagonistic assay on five different media. We observed 11 distinct types of pathogen inhibition encompassing a variable degree of antagonism on changing the media. This revealed the potential chemodiversity of the isolated fungal endophytes not only as promising resources of biocontrol agents against the known and emerging phytopathogens of Cannabis plants, but also as sustainable resources of biologically active and defensive secondary metabolites.
Not Cannabis specific
Engineering Monoterpene Production in Yeast Using a Synthetic Dominant Negative Geranyl Diphosphate Synthase.
Ignea, C., Pontini, M., Maffei, M. E., Makris, A. M., & Kampranis, S. C
ACS Synthetic Biology, 3(5), 298-306. (2014).
doi:10.1021/sb400115e
Monoterpenes have an established use in the food and cosmetic industries and have recently also found application as advanced biofuels. Although metabolic engineering efforts have so far achieved significant yields of larger terpenes, monoterpene productivity is lagging behind. Here, we set out to establish a monoterpene-specific production platform in Saccharomyces cerevisiae and identified the sequential reaction mechanism of the yeast farnesyl diphosphate synthase Erg20p to be an important factor limiting monoterpene yield. To overcome this hurdle, we engineered Erg20p into a geranyl diphosphate synthase and achieved a significant increase in monoterpene titers. To further improve production, we converted the engineered geranyl diphosphate synthase into a dominant negative form, so as to decrease the ability of the endogenous Erg20p to function as a farnesyl diphosphate synthase, without entirely abolishing sterol biosynthesis. Fusion of the synthetic dominant negative Erg20p variant with the terpene synthase, combined with yeast strain engineering, further improved monoterpene yields and achieved an overall 340-fold increase in sabinene yield over the starting strain. The design described here can be readily incorporated to any dedicated yeast strain, while the developed plasmid vectors and heterozygous ERG20 deletion yeast strain can also be used as a plug-and-play system for enzyme characterization and monoterpene pathway elucidation
Essential oils from Cannabis sativa L.
Alessandro Zatta
September 2005 Conference: 36th International Symposium on Essential Oils. At: Budapest (H)
FIND LINK OR DOI then list on IC
https://www.researchgate.net/publica...nabis_sativa_L
Hemp (Cannabis sativa L.) is an annual species, native of central Asia and spread in Europe and Africa, source of hundreds of biological active compounds such as cannabinoids, terpenoids, flavonoids and polyunsaturated fatty acids. Hemp essential oil, with its unique smell is, at present, used in cosmetic and perfume products, aromatherapy and as beer flavouring agent. Moreover it is traditionally employed as anti-inflammatory in the respiratory and digestive tracts and some its components possess recognized biological properties. In particular, myrcene is a potent analgesic (Rao et al., 1990), 1,8-cineole increases cerebral blood flow and enhances cortical activity (Nasel et al., 1994)and limonene inhibits the growth of many species of fungi and bacteria and as well as -pinene, -terpineol and borneol possesses repellent effects against many insects. For these aspects, it has the potential to be more exploited in different applications. In our research, five hemp cultivars, three dioic (Carmagnola, Dioica 88 and Fibranova ) and two monoic ( Epsilon and Futura), cultivated as fibre crops were also evaluated for the essential oil yields and compositions of their inflorescences. The aim of this work was to deep the knowledge on hemp essential oils and to obtain preliminary information on the possible exploitation the inflorescences, unused in fibre production, as source of a value-added for these cultivars. The oils, obtained by steam distillation and characterized by GC-MS, were made up by the same pool of components with ?-pinene ( from 9.5 to 16.3%), myrcene (from 14.6 to 20.9%) and ?-caryophyllene (from 10.3 to 24.6%) as main constituents followed by ?-pinene, limonene, trans ocimene, terpinolene and ?-humulene. The cultivars showed marked quantitative differences. The oil from Carmagnola had the highest content of myrcene, that from Fibranova was characterized by the higher amounts of ?-caryophyllene and ??humulene and that from Epsilon was the richest in ?-pinene and terpinolene and possessed also a high content of myrcene. The cultivar Fibranova showed the highest oil yield (0.28%) whereas Epsilon the lowest one (0.13%).
Essential Oil of Cannabis sativa L: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes
Ylenia Pieracci, Roberta Ascrizzi, Valentina Terreni, Luisa Pistelli, Guido Flamini, Laura Bassolino, Flavia Fulvio, Massimo Montanari and Roberta Paris
Molecules 2021, 26, 4080.
DOI: 10.3390/ molecules26134080
Cannabis sativa L. is an annual species cultivated since antiquity for different purposes. While, in the past, hemp inflorescences were considered crop residues, at present, they are regarded as valuable raw materials with different applications, among which extraction of the essential oil (EO) has gained increasing interest in many fields. The aim of the present study is the evaluation of the yield and the chemical composition of the EO obtained by hydrodistillation from eleven hemp genotypes, cultivated in the same location for two consecutive growing seasons. The composition of the EOs was analyzed by GC–MS, and then subjected to multivariate statistical analysis. Sesquiterpenes represented the main class of compounds in all the EOs, both in their hydrocarbon and oxygenated forms, with relative abundances ranging from 47.1 to 78.5%; the only exception was the Felina 32 sample collected in 2019, in which cannabinoids predominated. Cannabinoids were the second most abundant class of compounds, of which cannabidiol was the main one, with relative abundances between 11.8 and 51.5%. The statistical distribution of the samples, performed on the complete chemical composition of the EOs, evidenced a partition based on the year of cultivation, rather than on the genotype, with the exception of Uso-31. Regarding the extraction yield, a significant variation was evidenced among both the genotypes and the years of cultivation.
Essential oil of Cannabis sativa L. strains
Vito Mediavilla and Simon Steinemann
Journal of the International Hemp Association Vol.4 Issue 2 1997
https://www.internationalhempassocia.. ./jiha4208.html
The aroma of hemp (Cannabis sativa L.) could be of considerable commercial value if evaluation of varieties and development of extraction methods led to a pleasing scent in the resulting essential oils. We compared the composition and smell of some fiber hemp and drug Cannabis essential oils isolated by steam distillation. The essential oil of some hemp strains contained particular monoterpenes and sesquiterpenes that imparted to the specimen a desireable scent. These preliminary one-year results do not take into account the influence that harvest time and the weather "just-before-harvest" could have on the quality of the essential oil. The ?9-tetrahydrocannabinol (THC) concentration in the essential oils was very low and varied between 0.02% and 0.08%. The ratio of this compound to cannabidiol showed only small changes during steam distillation.
Not Cannabis specific
Essential Oil Safety
A Guide for Health Care Professionals
Robert Tisserand, Rodney Young, Elizabeth M Williamson
S E C O N D E D I T I O N
DOI: 10.1016/B978-0-443-06241-4.00001-1
This revised edition took 12 years to complete, and is considerably longer than the previous edition. There are three reasons for the comprehensive revision. First, since the text was first published in 1995, there have been many notable developments in the area of essential oil safety. In addition to new data being published, many guidelines and restrictions have been revised or issued by various authorities, and we have introduced some of
our own. Second, significant changes and improvements have been made to the text, especially in the area of profiles, some of these in response to reader feedback. The structure of both the Essential Oil Profiles and the Constituent Profiles has been considerably elaborated, and new material has been added. This edition includes 400 Essential Oil Profiles, compared to 95 previously. For each essential oil there is a full breakdown of constituents, and a clear categorization of hazards and risks, with recommended maximum doses and concentrations. All the compositional data for essential oils has been revised, expanded and referenced. There are 206 Constituent Profiles, and this section is 15 times that of the previous edition. Constituents are cross-referenced: each Constituent Profile lists the amount of that substance found in each of the 400 profiled essential oils. Third, the structure of the book has been developed. There are now separate chapters on the nervous, urinary, cardiovascular, gastrointestinal, and respiratory systems. Some sections of
text have moved from one chapter to another, and repetitive or outdated material has been deleted. We now have detailed safety advice on drug interactions, and overall there are more cautions. The new material is reflected in over 3,400 new references. A number of minor changes have also been made, such as the styling of references and the categorization of
constituents.
Not Cannabis specific
EssOilDB: A database of essential oils reflecting terpene composition and variability in the plant kingdom
Sachin Pundhir, Ganga Jeena
DOI: 10.1093/database/bau120
Database The Journal of Biological Databases and Curation · January 2014
Plant essential oils are complex mixtures of volatile organic compounds, which play indispensable roles in the environment, for the plant itself, as well as for humans. The potential biological information stored in essential oil composition data can provide an insight into the silent language of plants, and the roles of these chemical emissions in defense, communication and pollinator attraction. In order to decipher volatile profile patterns from a global perspective, we have developed the ESSential OIL DataBase
(EssOilDB), a continually updated, freely available electronic database designed to provide knowledge resource for plant essential oils, that enables one to address a multitude of queries on volatile profiles of native, invasive, normal or stressed plants, across taxonomic clades, geographical locations and several other biotic and abiotic influences.
To our knowledge, EssOilDB is the only database in the public domain providing an opportunity for context based scientific research on volatile patterns in plants. EssOilDB presently contains 123 041 essential oil records spanning a century of published reports on volatile profiles, with data from 92 plant taxonomic families, spread across diverse geographical locations all over the globe. We hope that this huge repository of VOCs will facilitate unraveling of the true significance of volatiles in plants, along with creating potential avenues for industrial applications of essential oils. We also illustrate the use of this database in terpene biology and show how EssOilDB can be used to complement data from computational genomics to gain insights into the diversity and variability of terpenoids in the plant kingdom. EssOilDB would serve as a valuable information resource, for students and researchers in plant biology, in the design and discovery of new odor profiles, as well as for entrepreneurs—the potential for generating consumer specific scents being one of the most attractive and interesting topics in the cosmetic industry.
Ethephon application stimulats cannabinoids and plastidic terpenoids production in Cannabis sativa at flowering stage
Hakimeh Mansouri, , Fatemeh Salari, Zahra Asrar
Industrial Crops and Products
doi: 10.1016/j.indcrop.2013.01.025
We studied the effect of ethephon on levels of the major cannabinoids (tetrahydrocannabinol and cannabidiol) and chlorophyll, carotenoids and ?-tocopherol in Cannabis sativa at productive stage. Results revealed that ethephon increased THCcontent of leaf in male and female plants and of male flowers. However, ethephon unable to enhancing THC content in female flowers. Treatment with etheohon increased CBD content in male and female leaf and female flowers. The treatment of male flowers with low ethephon concentration caused an increase, and those treated with high ethephon concentration resulted in a decrease in CBD content. The lowest level of ethephon (1 ?M) enhanced chlorophyll a, b and total chlorophyll in male and female plants. Both sexes treated with ethephon showed an increase in carotenoids content, but 1 ?M ethephon had the stronger effect in this regards. Male and female plants had a higher content of ?-tocopherol when treated with ethephon. These results showed ethephon is a suitable treatment for increasing cannabinoids and ?-tocopherol in productive stage of cannabis and there was not a relation between primary and secondary terpenoids.
? Ethephon treatment has considerable effects on increasing of cannabinoids in male and female cannabis. ? There is no correlation between cannabinoids and other plastidial terpenoids. ? Using of ethephon treatment in flowering stage do not adverse effect on plant growth.
ERRATUM
Evaluation of Cannabinoid and Terpenoid Content: Cannabis Flower Compared to Supercritical CO2 Concentrate
Michelle Sexton, Kyle Shelton, Pam Haley, Mike West
Planta Med, advance online publication September 19, 2017
doi:10.1055/s-0043-119361
? Table 1 was replaced by a new table.
In ? Tables 2 and 3 microgram/gram (?g/g) was modified
to mg/g.
? Fig. 2 was replaced by a new figure (in the figure microgram/
gram [?g/g] was modified to mg/gram).
The ?-caryophyllene result on page 3 in the section “Results”
was corrected, the factor is “5.1”.
Evaluation of cannabinoid and terpenoid content: Cannabis flower compared to supercritical CO2 concentrate.
Sexton M, Shelton K, Haley P, West M (2018)
Planta Med 84: 234–241
doi: 10.1055/s-0043-119361
A recent cannabis use survey revealed that 60% of cannabis users rely on smelling the flower to select their cannabis. Olfactory indicators in plants include volatile compounds, principally represented by the terpenoid fraction. Currently, medicinal- and adult-use cannabis is marketed in the United States with relatively little differentiation between products other than by a common name, association with a species type, and ?-9 tetrahydrocannabinol/cannabidiol potency. Because of this practice, how terpenoid compositions may change during an extraction process is widely overlooked. Here we report on a comparative study of terpenoid and cannabinoid potencies of flower and supercritical fluid CO2 (SC?CO2) extract from six cannabis chemovars grown in Washington State. To enable this comparison, we employed a validated high-performance liquid chromatography/diode array detector methodology for quantification of seven cannabinoids and developed an internal gas chromatography-mass spectrometry method for quantification of 42 terpenes. The relative potencies of terpenoids and cannabinoids in flower versus concentrate were significantly different. Cannabinoid potency increased by factors of 3.2 for ?-9 tetrahydrocannabinol and 4.0 for cannabidiol in concentrates compared to flower. Monoterpenes were lost in the extraction process; a ketone increased by 2.2; an ether by 2.7; monoterpene alcohols by 5.3, 7 and 9.4; and sesquiterpenes by 5.1, 4.2, 7.7, and 8.9. Our results demonstrate that the product of SC?CO2 extraction may have a significantly different chemotypic fingerprint from that of cannabis flower. These results highlight the need for more complete characterization of cannabis and associated products, beyond cannabinoid content, in order to further understand health-related consequences of inhaling or ingesting concentrated forms.
Evaluation of the terpenes β-caryophyllene, α-terpineol, and γ-terpinene in the mouse chronic constriction injury model of neuropathic pain: possible cannabinoid receptor involvement
Joshua A Bilbrey, Yuma T Ortiz, Jasmine S Felix, Lance R McMahon, Jenny L Wilkerson
Psychopharmacology . 2021 Nov 30.
doi: 10.1007/s00213-021-06031-2
Pain is one of the most common reasons to seek medical attention, and chronic pain is a worldwide epidemic. Anecdotal reports suggest cannabis may be an effective analgesic. As cannabis contains the terpenes α-terpineol, β-caryophyllene, and γ-terpinene, we hypothesized these terpenes would produce analgesia in a mouse model of neuropathic pain. We used the chronic constriction injury of the sciatic nerve mouse model, which produces mechanical allodynia, assessed via the von Frey assay, as well as thermal hyperalgesia assessed via the hotplate assay. Compounds were further assessed in tests of locomotor activity, hypothermia, and acute antinociception. Each terpene produced dose-related reversal of mechanical allodynia and thermal hyperalgesia. Thermal hyperalgesia displayed higher sensitivity to the effects of each terpene than mechanical allodynia, and the rank order potency of the terpenes was α-terpineol > β-caryophyllene > γ-terpinene. To examine the involvement of cannabinoid receptors, further tests were conducted in mice lacking either functional cannabinoid type 1 receptors (CB1R (-/-)) or cannabinoid type 2 receptors (CB2R (-/-)). Compared to wild type mice, CB1R (-/-) mice treated with α-terpineol displayed a 2.91-fold decrease in potency to reverse mechanical allodynia; in CB2R (-/-) mice, the potency of α-terpineol was decreased 11.73-fold. The potency of β-caryophyllene to reverse mechanical allodynia decreased 1.80-fold in CB2R (-/-) mice. Each terpene produced a subset of effects in tests of locomotor activity, hypothermia, and acute antinociception. These findings suggest α-terpineol, β-caryophyllene, and γ-terpinene may have differential cannabinoid receptor activity and a pharmacological profile that may yield new efficacious analgesics.
Find Pdf
Not Cannabis Specific
The Science Behind Essential OilsResearch
EOU maintains an active role in essential oil research, here are some published research by EOU staff on essential oils.essentialoils.org
We maintain an active role in the research of essential oils on various levels. Dr. Pappas regularly submits publications to reputable scientific journals like Journal of Essential Oil Research (JEOR) on the chemical properties of unusual essential oils as well as submitting articles relevant to aromatherapy to journals like Aromatherapy Journal (formerly Scentsitivity, published by the National Association for Holistic Aromatherapy). Dr. Pappas has also been involved with essential oil and aromatherapy education at local colleges and universities.
Some Scientific Journal Publications by Dr. Pappas related to essential oils. We hope you enjoy them
Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes
Oier Aizpurua-Olaizola†‡, Umut Soydaner†, Ekin Öztürk†, Daniele Schibano†, Yilmaz Simsir†, Patricia Navarro‡, Nestor Etxebarria‡, and Aresatz Usobiaga*‡
J. Nat. Prod.
DOI: 10.1021/acs.jnatprod.5b00949
The evolution of major cannabinoids and terpenes during the growth of Cannabis sativa plants was studied. In this work, seven different plants were selected: three each from chemotypes I and III and one from chemotype II. Fifty clones of each mother plant were grown indoors under controlled conditions. Every week, three plants from each variety were cut and dried, and the leaves and flowers were analyzed separately. Eight major cannabinoids were analyzed via HPLC-DAD, and 28 terpenes were quantified using GC-FID and verified via GC-MS. The chemotypes of the plants, as defined by the tetrahydrocannabinolic acid/cannabidiolic acid (THCA/CBDA) ratio, were clear from the beginning and stable during growth. The concentrations of the major cannabinoids and terpenes were determined, and different patterns were found among the chemotypes. In particular, the plants from chemotypes II and III needed more time to reach peak production of THCA, CBDA, and monoterpenes. Differences in the cannabigerolic acid development among the different chemotypes and between monoterpene and sesquiterpene evolution patterns were also observed. Plants of different chemotypes were clearly differentiated by their terpene content, and characteristic terpenes of each chemotype were identified.
Expression and characterization of terpene synthases from Cannabis sativa L. and Salvia sclarea L.
Nils Gu?nnewich THESIS
https://www.researchgate.net/publica...lvia_sclarea_L
Many volatile oil (or essential) oil-producing plants are particular interesting because of their high economic value. The term ‘volatile oil’ is preferred because it refers to the fact that most components of the oils, which are stored in extra cellular spaces in the epidermis or mesophyll, have low boiling points and can be recovered from the plant tissues by steam distillation. Volatile oils are quite distinct from more common triglyceride oils and fats, which are known as ‘fixed’, because of their high boiling point [1]. Vast amounts of volatile oils are terpenoids that are made up of five-carbon isoprene or isopentenoid units. Other biological molecules belong to this family, are for example; porphyrins, chlorophylls, carotenoids, steroids, gibberellins and natural rubber [2]. The production and accumulation of volatile, low molecular weight terpenes [mono-(C10), most sesqui-(C15), and some diterpenes (C20)] are not restricted to one specialized taxonomic group but occur throughout the plant kingdom. Volatile oils can be obtained from various tissues of Gymnosperms (Pinaceae: conifers; Taxaceae: the yew family; Cupressaceae: cypresses and junipers; Cycadaceae: the tree fern family). The ability to accumulate terpenes is widely distributed in Angiosperms (subphylum: Magnoliophytina), too. By temperate and tropical dicotyledons (Chenopodiaceae: beets and goosefoots; Compositae: the daisy family; Geraniaceae: the geranium family; Guttiferae/Hypericaceae: including St. Johns worts; Lamiaceae: the mint family; Cannabaceae: hemp and hop; Lauraceae: including bays; Myricacea; Myristicaceae: nutmeg and mace; Myrtaceae: myrtles, eucalypts and clove; Scrophulariaceae: olives and lilacs; Piperaceae: the pepper family; Rosaceae: the rose family; Rutaceae: the citrus family; Santalaceae: sandalwood; Apiaceae: the carrotfamily; Verbenaceae: including verbenas; Violaceae: violets and pansies) and monocotyledons (Araceae: the aroid family; Cyperaceae: the sedge family; Poaceae: the grass family; Zingiberaceae: the ginger family). This indicates that the accumulation of terpenes was a feature of the earliest seed plants [1]. In this doctoral thesis two volatile oil producing plants were investigated, due to their ability to produce economically valuable terpenoids, Cannabis sativa L. and Salvia sclarea L..
Extraction of Phenolic Compounds and Terpenes from Cannabis sativa L. By-Products: From Conventional to Intensified Processes
Emilie Isidore, Hamza Karim, Irina Ioannou
https://www.researchgate.net/publica...fied_Processes
Cannabis sativa L. is a controversial crop due to its high tetrahydrocannabinol content varieties; however, the hemp varieties get an increased interest. This paper describes (i) the main categories of phenolic compounds (flavonoids, stilbenoids and lignans) and terpenes (monoterpenes and sesquiterpenes) from C. sativa by-products and their biological activities and (ii) the main extraction techniques for their recovery. It includes not only common techniques such as conventional solvent extraction, and hydrodistillation, but also intensification and emerging techniques such as ultrasound-assisted extraction or supercritical CO2 extraction. The effect of the operating conditions on the yield and composition of these categories of phenolic compounds and terpenes was discussed. A thorough investigation of innovative extraction techniques is indeed crucial for the extraction of phenolic compounds and terpenes from cannabis toward a sustainable industrial valorization of the whole plant.
Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil
C. Meier, Vito Mediavilla
January 1998
https://www.internationalhempassocia.. ./jiha5107.html
https://therichardrosereport.com/dow...essential-oil/
The aim of this work was to assess the factors influencing the yield and the quality of hemp essential oil. Several strains were used to carry out field and greenhouse experiments, and as specimens for scent tests and chemical analysis of the distilled oils.
Yields of oil were highest at seeding rates of 5 kg/ha and when about a 50% of the crop had reached maturity. Pollination led to significantly lower yields, but is not easy to prevent in the field. Chemical composition showed almost no relationship to harvest dates or to scores on the scent tests. The best scent quality was always obtained from plants harvested one to three weeks before seed maturity (75% of seed matured). The interval during which both yield and quality were high was rather short and had to be assessed for each strain. The influence of other factors such as weather and harvest technique were also evaluated.
Fast, Accurate, and Precise Terpene Testing of Cannabis Samples
Lee Marotta, David Scott, Adam Floyd, Toby Astill, Cassandra (Cassie) Ereman, Ben Armstrong
PerkinElmer
https://www.perkinelmer.com/lab-solu..._014579_01.pdf
Like all botanicals and plants found in nature, cannabis also contains terpenes, which are the aromatic oils that give rise to the distinctive flavors and aromas found in cannabis varieties. There have been up to 140 different types of terpenes reported in cannabis, but multiple studies suggest that approximately 17 are the most common and can be used for examining their chemotype (chemotype: those strains that have chemical properties that differ from each other’s). Among them are monoterpenes, diterpenes, and sesquiterpenes, which are characterized by the number of repeating units of a five-carbon molecule, called isoprene, the structural hallmark of all terpenoid compounds. The diverse palate of cannabis terpenes is impressive enough, but arguably their most fascinating characteristic is their ability to interact synergistically with other compounds in the plant, like cannabinoids. In the past few decades, a significant amount of work has been performed to understand the ‘entourage effect’, which scientists refer to as synergistic interaction between terpenes and cannabinoids in the human body. This effect is believed to magnify the therapeutic benefits of the plant’s individual components — so that the medicinal impact of the whole plant is greater than the sum of its parts quantifying which terpenes are present is an important aspect of understanding the unique effects of cannabis for both medicinal and recreational users.
Fibre hemp inflorescences: From crop-residues to essential oil production
Alessandra Bertoli, Sabrina Tozzi, Luisa Pistelli, Luciana G. Angelini
Industrial Crops and Products 32 (2010) 329–337
doi: 10.1016/j.indcrop.2010.05.012
The volatile composition of ten fibre hemp (Cannabis sativa L.) varieties was investigated during two successive growing seasons under temperate climatic conditions in Central Italy.
The freshly plant inflorescences were hydrodistilled and the essential oils (EOs) were characterized by GC–MS. In addition, the composition of the aroma emitted spontaneously from the freshly plant inflorescences were analysed by SPME-GC–MS. The EO yields of eight dioecious (Carmagnola, C.S., Red Petiole, Pop 1, Pop 2, Pop 3, Pop 4, Pop 5) and two monoecious (Codimono and Felina 34) cultivars ranged from 0.11 to 0.25% (w/w) and showed a significant production of a-pinene (3–20%), b-pinene (1–8%), E-ocimene (1–10%), myrcene (8–45%) and terpinolene (0.12–22%).
The monoterpene composition was useful to distinguish the monoecious cultivars from the dioecious ones. b-Caryophyllene (7–28%), a-humulene (3–12%), and caryophyllene oxide (2–6%) were the main sesquiterpenes. Tetrahydrocannabinol (THC) was present in traces in the EOs of only two dioecious cultivars cultivated in 2005. Cannabinol (CBN) was not detected in the essential oils, while the no-hallucinogenous cannabidiol (CBD) was found as typical volatile constituent in several analysed cultivars. These findings were also confirmed by the headspace GC–MS analysis carried out on the same samples. The analysed EOs obtained from fibre hemp varieties cultivated in Central Italy were characterized by an interesting and specific terpene composition with a legal and safe cannabinoid content. They were obtained from freshly plant inflorescences, which usually represent a waste material from C. sativa L. fibre varieties. The present study strengths the hypothesis to grow hemp as a multi-use crop through a complete utilization of the plant material using inflorescences to produce essential oils as natural flavour and fragrance additives.
NOT CANNABIS SPECIFIC
Four terpene synthases contribute to the generation of chemotypes in tea tree (Melaleuca alternifolia)
Amanda Padovan, Andras Keszei, Yasmin Hassan, Sandra T. Krause, Tobias G. Köllner, Jörg Degenhardt, Jonathan Gershenzon, Carsten Külheim and William J. Foley
BMC Plant Biology (2017) 17:160
DOI 10.1186/s12870-017-1107-2
Background: Terpene rich leaves are a characteristic of Myrtaceae. There is significant qualitative variation in the terpene profile of plants within a single species, which is observable as “chemotypes”. Understanding the molecular basis of chemotypic variation will help explain how such variation is maintained in natural populations as well as allowing focussed breeding for those terpenes sought by industry. The leaves of the medicinal tea tree, Melaleuca alternifolia, are used to produce terpinen-4-ol rich tea tree oil, but there are six naturally occurring chemotypes; three cardinal chemotypes (dominated by terpinen-4-ol, terpinolene and 1,8-cineole, respectively) and three intermediates. It has been predicted that three distinct terpene synthases could be responsible for the maintenance of chemotypic variation in this species.
Results: We isolated and characterised the most abundant terpene synthases (TPSs) from the three cardinal chemotypes of M. alternifolia. Functional characterisation of these enzymes shows that they produce the dominant compounds in the foliar terpene profile of all six chemotypes. Using RNA-Seq, we investigated the expression of these and 24 additional putative terpene synthases in young leaves of all six chemotypes of M. alternifolia. Conclusions: Despite contributing to the variation patterns observed, variation in gene expression of the three TPS genes is not enough to explain all variation for the maintenance of chemotypes. Other candidate terpene synthases as well as other levels of regulation must also be involved. The results of this study provide novel insights into the complexity of terpene biosynthesis in natural populations of a non-model organism.
Not Cannabis Specific
Functional analysis of (4S)-limonene synthase mutants reveals determinants of catalytic outcome in a model monoterpene synthase
Narayanan Srividya, Edward M. Davis, Rodney B. Croteau1 , and B. Markus Lange
PNAS | March 17, 2015 | vol. 112 | no. 11
DOI: 10.1073/pnas.1501203112
Crystal structural data for (4S)-limonene synthase [(4S)-LS] of spearmint (Mentha spicata L.) were used to infer which amino acid residues are in close proximity to the substrate and carbocation intermediates of the enzymatic reaction. Alanine-scanning mutagenesis of 48 amino acids combined with enzyme fidelity analysis [percentage of (?)-limonene produced] indicated which residues are most likely to constitute the active site. Mutation of residues W324 and H579 caused a significant drop in enzyme activity and formation of products (myrcene, linalool, and terpineol) characteristic of a premature termination of the reaction. A double mutant (W324A/H579A) had no detectable enzyme activity, indicating that either substrate binding or the terminating reaction was impaired. Exchanges to other aromatic residues (W324H, W324F, W324Y, H579F, H579Y, and H579W) resulted in enzyme catalysts with significantly reduced activity. Sequence comparisons across the angiosperm lineage provided evidence that W324 is a conserved residue, whereas the position equivalent to H579 is occupied by aromatic residues (H, F, or Y). These results are consistent with a critical role of W324 and H579 in the stabilization of carbocation intermediates. The potential of these residues to serve as the catalytic base facilitating the terminal deprotonation reaction is discussed.
Significance: Terpene synthases catalyze complex, chain length-specific, electrophilic cyclization reactions that constitute the first committed step in the biosynthesis of structurally diverse terpenoids. (4S)-limonene synthase [(4S)-LS] has emerged as a model enzyme for enhancing our comprehension of the reaction cycle of monoterpene (C10) synthases. While the stereochemistry of the cyclization of geranyl diphosphate to (?)-(4S)-limonene has been the subject of several mechanistic studies, the structural basis for the stabilization of carbocation intermediates and the termination of the reaction sequence have remained enigmatic. We present extensive experimental evidence that the aromatic amino acids W324 and H579 play critical roles in the stabilization of intermediate carbocations. A possible function of these residues as the terminal catalytic base is also discussed.
Functional expression and characterization of trichome-specific (-)-limonene synthase and (+)-a-pinene synthase from Cannabis sativa
Nils Gu?nnewich,Jonathan E. Page, Tobias G. Köllner, Jörg Degenhardt, Toni M. Kutchana
Natural product communications · March 2007 Natural Product Communications Vol. 0 (0) 2006
https://www.researchgate.net/profile...bis-sativa.pdf
Two recombinant, stereospecific monoterpene synthases, a (-)-limonene synthase (CsTPS1) and a (+)-?-pinene synthase (CsTPS2), encoded by Cannabis sativa L. cv. ‘Skunk’ trichome mRNA, have been isolated and characterized. Recombinant CsTPS1 shows a Km value at 6.8 ?M and a Kcat at 8.2 x 10-2 s-1, the pH optimum was determined at pH 6.5, and a temperature optimum at 40°C. Recombinant CsTPS2 shows a Km values at 6.7 ?M and a Kcat at 8.1 x 10-2 s-1, the pH optimum was determined at pH 7.0, and a temperature optimum at 30°C. Phylogenetic analysis showed that both CsTPSs group within the angiosperm family and belong to the Tpsb subgroup of monoterpene synthases. The enzymatic products (-)-limonene and (+)-?-pinene were detected as natural products in C. sativa trichomes.
NOT CANNABIS SPECIFIC
Genetic Control and Evolution of Sesquiterpene Biosynthesis in Lycopersicon esculentum and L. hirsutum
Rutger S. van der Hoeven, Antonio J. Monforte, David Breeden, Steven D. Tanksley, and John C. Steffens
The Plant Cell, Vol. 12, 2283–2294, November 2000,
DOI: 10.1105/tpc.12.11.2283
Segregation analysis between Lysopersicon esculentum (cultivated tomato) and L. hirsutum (wild form) in conjunction with positional verification by using near-isogenic lines demonstrated that biosynthesis of two structurally different classes of sesquiterpenes in these species is controlled by loci on two different chromosomes. A locus on chromosome 6, Sesquiterpene synthase 1 (Sst1), was identified for which the L. esculentum allele is associated with the biosynthesis of b-caryophyllene and a-humulene. At this same locus, the L. hirsutum allele is associated with biosynthesis of germacrene B, germacrene D, and an unidentified sesquiterpene. Genomic mapping, cDNA isolation, and heterologous expression of putative sesquiterpene synthases from both L. esculentum and L. hirsutum revealed that Sst1 is composed of two gene clusters 24 centimorgans apart, Sst1-A and Sst1-B, and that only the genes in the Sst1-A cluster are responsible for accumulation of chromosome 6–associated sesquiterpenes. At a second locus, Sst2, on chromosome 8, the L. hirsutum allele specified accumulation of a-santalene, a-bergamotene, and b-bergamotene. Surprisingly, the L. esculentum allele for Sst2 is not associated with the expression of any sesquiterpenes, which suggests that cultivated tomato may have a nonfunctional allele. Sesquiterpene synthase cDNA clones on chromosome 6 do not cross-hybridize on genomic DNA gel blots with putative sesquiterpene synthases on chromosome 8, an indication that the genes in Sst1 and Sst2 are highly diverged, each being responsible for the biosynthesis of structurally different sets of sesquiterpenes.
Not Cannabis specific Genetic Engineering of Terpenoid Metabolism Attracts Bodyguards to Arabidopsis
Iris F. Kappers, Asaph Aharoni, Teun W. J. M. van Herpen, Ludo L. P. Luckerhoff, Marcel Dicke, Harro J. Bouwmeester
SCIENCE 23 SEPT 2005 VOL 309
DOI: 10.1126/science.1116232
Herbivore-damaged plants release complex mixtures of volatiles that attract
natural enemies of the herbivore. To study the relevance of individual components of these mixtures for predator attraction, we manipulated herbivoryinduced volatiles through genetic engineering. Metabolic engineering of terpenoids, which dominate the composition of many induced plant volatile bouquets, holds particular promise. By switching the subcellular localization of the introduced sesquiterpene synthase to the mitochondria, we obtained transgenic Arabidopsis thaliana plants emitting two new isoprenoids. These altered plants attracted carnivorous predatory mites (Phytoseiulus persimilis) that aid the plants’ defense mechanisms
*Genomic characterization of the complete terpene synthase gene family from Cannabis sativa.
Allen KD, McKernan K, Pauli C, Roe J, Torres A, Gaudino R
PLoS ONE 14 (9): e0222363. (2019)
Doi: 10.1371/journal. pone.0222363
Terpenes are responsible for most or all of the odor and flavor properties of Cannabis sativa, and may also impact effects users experience either directly or indirectly. We report the diversity of terpene profiles across samples bound for the Washington dispensary market. The remarkable degree of variation in terpene profiles ultimately results from action of a family of terpene synthase genes, only some of which have been described. Using a recently available genome assembly we describe 55 terpene synthases with genomic context, and tissue specific expression. The family is quite diverse from a protein similarity perspective, and subsets of the family are expressed in all tissues in the plant, including a set of root specific monoterpene synthases that could well have agronomic importance. Ultimately understanding and breeding for specific terpene profiles will require a good understanding of the gene family that underlies it. We intend for this work to serve as a foundation for that.
Genomic Organization of Plant Terpene Synthases and Molecular Implications
Susan C Trapp, Rodney B Croteau
Genetics 158(2):811-32July 2001
DOI: 10.1093/genetics/158.2.811
https://www.researchgate.net/publica...r_Implications
Terpenoids are the largest, most diverse class of plant natural products and they play numerous functional roles in primary metabolism and in ecological interactions. The first committed step in the formation of the various terpenoid classes is the transformation of the prenyl diphosphate precursors, geranyl diphosphate, farnesyl diphosphate, and geranylgeranyl diphosphate, to the parent structures of each type catalyzed by the respective monoterpene (C(10)), sesquiterpene (C(15)), and diterpene synthases (C(20)). Over 30 cDNAs encoding plant terpenoid synthases involved in primary and secondary metabolism have been cloned and characterized. Here we describe the isolation and analysis of six genomic clones encoding terpene synthases of conifers, [(-)-pinene (C(10)), (-)-limonene (C(10)), (E)-alpha-bisabolene (C(15)), delta-selinene (C(15)), and abietadiene synthase (C(20)) from Abies grandis and taxadiene synthase (C(20)) from Taxus brevifolia], all of which are involved in natural products biosynthesis. Genome organization (intron number, size, placement and phase, and exon size) of these gymnosperm terpene synthases was compared to eight previously characterized angiosperm terpene synthase genes and to six putative terpene synthase genomic sequences from Arabidopsis thaliana. Three distinct classes of terpene synthase genes were discerned, from which assumed patterns of sequential intron loss and the loss of an unusual internal sequence element suggest that the ancestral terpenoid synthase gene resembled a contemporary conifer diterpene synthase gene in containing at least 12 introns and 13 exons of conserved size. A model presented for the evolutionary history of plant terpene synthases suggests that this superfamily of genes responsible for natural products biosynthesis derived from terpene synthase genes involved in primary metabolism by duplication and divergence in structural and functional specialization. This novel molecular evolutionary approach focused on genes of secondary metabolism may have broad implications for the origins of natural products and for plant phylogenetics in general.
Not Cannabis specific
Geraniol Restores Antibiotic Activities against Multidrug-Resistant Isolates from Gram-Negative Species.
Lorenzi, V., Muselli, A., Bernardini, A. F., Berti, L., Pages, J.-M., Amaral, L., & Bolla, J.-M.
Antimicrobial Agents and Chemotherapy, 53(5), 2209–2211.(2009).
doi:10.1128/aac.00919-08
The essential oil of Helichrysum italicum significantly reduces the multidrug resistance of Enterobacter aerogenes, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii. Combinations of the two most active fractions of the essential oil with each other or with phenylalanine arginine -naphthylamide yield synergistic activity. Geraniol, a component of one fraction, significantly increased the efficacy of -lactams, quinolones, and chloramphenicol.
Got Terps? Cannabis Extracts Lack the Same Compounds as Flower
Chris Roberts
https://cannabisnow.com/got-terps-ca...nds-as-flower/
It can be easy to convince yourself that concentrated cannabis is cannabis, just more of it. You can argue that concentrates have more of the good stuff and less carbon-based plant material: More terpenes, more THC, more bounce to the ounce (or bam to the gram, whatever). Just taste that surge of limonene and feel the impact on your brain and body as that wave of 80% THC crashes over your consciousness and try to say otherwise!
But contrary to what your senses might tell you about concentrate superiority, science has spoken. And science says the extraction process, as sophisticated as it may be, removes significant active ingredients from the plant-based source material — including the material that, for most consumers, determines what strain is their favourite.
Grinding and Fractionation during Distillation Alter Hemp Essential Oil Profile and Its Antimicrobial Activity.
Zheljazkov, V. D., Sikora, V., Semerdjieva, I. B., Kačániová, M., Astatkie, T., & Dincheva, I.
Molecules, 25(17), 3943.(2020).
doi:10.3390/molecules25173943
The hypothesis of this study was that we can modify the essential oil (EO) profile of hemp (Cannabis sativa L.) and obtain fractions with differential composition and antimicrobial activity. Therefore, the objective was to evaluate the effects of grinding of hemp biomass before EO extraction and fractionation during distillation on EO profile and antimicrobial activity. The study generated a several EO fractions with a diversity of chemical profile and antimicrobial activity. The highest concentrations of β-pinene and myrcene in the EO can be obtained in the 5–10 min distillation time (DT) of ground material or in the 80–120 min DT of nonground material. High δ-3-carene and limonene EO can be obtained from 0–5 min DT fraction of nonground material. High eucalyptol EO can be sampled either in the 0–5 min DT of the ground material or in the 80–120 min of nonground material. Overall, the highest concentrations of β-caryophyllene, α-(E)-bergamotene, (Z)-β-farnesene, α-humulene, caryophyllenyl alcohol, germacrene D-4-ol, spathulenol, caryophyllene oxide, humulene epoxide 2, β-bisabolol, α-bisabolol, sesquiterpenes, and cannabidiol (CBD) can be obtained when EO is sampled in the 80–120 min DT and the material is nonground. Monoterpenes in the hemp EO can be increased twofold to 85% by grinding the material prior to distillation and collecting the EO in the first 10 min. However, grinding resulted in a slight but significant decrease in the CBD concentration of the EO. CBD-rich oil can be produced by collecting at 120–180 min DT. Different EO fractions had differential antimicrobial activity. The highest antimicrobial activity of EO fraction was found against Staphylococcus aureus subsp. aureus. THC-free EO can be obtained if the EO distillation is limited to 120 min. The results can be utilized by the hemp processing industry and by companies developing new hemp EO-infused products, including perfumery, cosmetics, dietary supplements, food, and pharmaceutical industries.
Not Cannabis Specific
Handbook of Essential Oils
Science, Technology, and Applications, Second Edition
Edited ByK. Husnu Can Baser, Gerhard Buchbauer
DOI: 10.1201/b19393
DOI: 10.1201/9781420063165
Find link
The second edition of Handbook of Essential Oils: Science, Technology, and Applications provides a much-needed compilation of information related to the development, use, and marketing of essential oils. It focuses particularly on the chemistry, pharmacology, and biological activities of essential oils, with contributions from a worldwide group of
Headspace Solid Phase Microextraction (HS SPME) Gas Chromatography Mass Spectrometric Analysis of the Volatile Constituents of Cannabis sativa L. From Kashmir
Manzoor A. Rather, Bilal A. Dar, Shahnawaz N. Sofi, Tauheeda Hassan, Nasir Ali, Ashiq H. Lone, Abdul S. Shawl, Wajahat A. Shah, M. A. Qurishi and Poonam Prakash
Journal of Pharmacy Research 2011,4(8),2651-2653
https://citeseerx.ist.psu.edu/viewdo...=rep1&type=pdf
Headspace Solid-phase micro extraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS) has been used to isolate and identify the volatile compounds from the leaves of Cannabis sativa growing in Kashmir. The analysis led to the identification of 17 volatile components constituting 94.8 % of the total identified components. The chemical composition of the SPME extract from the leaves of C. sativa comprised mainly of sesquiterpene hydrocarbons (64.3%), monoterpene hydrocarbons (18.4%) and alcohols (10.3%). The major components identified in the HS-SPME extract were trans-caryophyllene (36.9%), a-humulene (16.2%), a-pinene (10.7%), 3-hexen-1-ol-acetate (6.2%) and ß-pinene (4.2%). The current study is the first report involving rapid analysis of volatile components of C. sativa by HS-SPME.
Headspace SPME GC/MS Analysis of Terpenes in Cannabis
Katherine K. Stenerson
Merck
https://www.sigmaaldrich.com/technic...spme-gcms.html
A rapid method to identify cannabis terpenes for forensic and organoleptic applications
Cannabis sativa (cannabis or marijuana) contains over 100 different terpenes and terpenoids, including mono, sesqui, di and tri, as well as other miscellaneous compounds of terpenoid orgin.1 Terpenes give the plant distinct organoleptic properties and produce characteristic aromas when the buds are heated or vaporized.2 Although the terpene profile does not necessarily indicate geographic origin of a cannabis sample, it can be used in forensic applications to determine the common source of different samples.3 In addition, different cannabis strains have been developed which have distinct aromas and flavors, a result of the differing amounts of specific terpenes present
Human metabolism of α-pinene and metabolite kinetics after oral administration Lukas Schmidt & Thomas Göen Archives of Toxicology volume 91, pages677–687 (2017)
DOI 10.1007/s00204-015-1656-9
We studied the human in vivo metabolism and the elimination kinetics of α-pinene (αPN), a natural monoterpene which commonly occurs in the environment. Four volunteers were exposed to a single oral dose of 10 mg αPN. Each subject provided one pre-exposure and subsequently all post-exposure urine samples up to 24 h after administration. Additionally, blood samples were drawn hourly from two volunteers for 5 h. The analysis of the parent compound in blood was performed by a headspace GC–MS procedure, whereas the proposed αPN metabolites myrtenol (MYR) and cis- and trans-verbenol (cVER; tVER) were quantified in blood and urine using GC–PCI-MS/MS. Unknown metabolites were investigated using GC–PCI-MS full-scan analyses. The urinary concentration of the metabolites reached their maxima 1.6 h after exposure. Afterwards, they declined to the pre-exposure levels within the 24-h observation period with elimination half-lives of 1.5 h (MYR) and 1.6 h (cVER and tVER). The total eliminated amounts corresponded to 1.5 % (MYR), 5.6 % (cVER), and 4.1 % (tVER) of the orally applied dose. The GC–PCI-MS full-scan analyses identified three novel metabolites, of which one conforms to myrtenic acid (MYRA). A re-analysis of MYRA in urine showed maximum elimination 1.6 h after αPN ingestion, an elimination half-life of 1.4 h, and a share of the oral dose of 6.7 %. The study revealed that the human in vivo metabolism of αPN proceeds fast and elimination of metabolites takes places rapidly. The metabolism of αPN is dominated by extensive oxidation reactions at the methyl side-chains yielding in carboxylic acid structures as well as by allylic oxidation of the cyclohexenyl backbone, whereas predicted products of a double-bond oxidation were not detected.
Human metabolism of Δ3 carene and renal elimination of Δ3 caren 10 carboxylic acid (chaminic acid) after oral administration Lukas Schmidt · Vladimir N. Belov · Thomas Göen
Arch Toxicol DOI 10.1007/s00204-014-1251-5
https://sci-hub.se/10.1007/s00204-014-1251-5
We studied the human in vivo metabolism of Δ3 -carene (CRN), a natural monoterpene which commonly occurs in the human environment. Four healthy human volunteers were orally exposed to a single dose of 10 mg CRN. Each volunteer gave one urine sample before administration and subsequently collected each urine sample within 24 h after administration. The concentration of the proposed CRN metabolites Δ3 -caren-10-ol (CRN-10-OH), Δ3 -caren-10-carboxylic acid (chaminic acid, CRN-10- COOH), and Δ3 -caren-3,4-diol (CRN-3,4-OH) were determined using a very specific and sensitive GC–MS/ MS procedure. Other CRN metabolites were investigated using GC–PCI–MS Q1 scan analyses. CRN-10-COOH was detected in each urine sample with maximum concentration (113.0–1,172.9 µg L−1 ) 2–3 h after administration, whereas CRN-10-OH and CRN-3,4-OH were not detected in any of the samples. The renal excretion kinetics of CRN-10- COOH showed an elimination half-life of about 3 h. The cumulative excretion of CRN-10-COOH within 24 h after exposure correlated with about 2 % of the applied dose. The GC–PCI–MS Q1 scan analysis indicated several additional human CRN metabolites; thereof, six spectra enabled the prediction of the corresponding chemical structure. The results of the study indicate that CRN-10-COOH is a relevant product of the human in vivo metabolism of CRN. The oxidation of its allylic methyl group proceeds until the acidic structure without interruption. Thus, the generation of the alcoholic intermediate appeared to be the rate-determining step of this metabolic route. Nevertheless, the proportion of CRN-10-COOH in the CRN metabolism is low, and other oxidative metabolites are likely. This hypothesis was confirmed by the discovery of additional human CRN metabolites, whose predicted chemical structures fit in with further oxidative products of CRN metabolism.
*Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive TwoDimensional Gas Chromatography
Iain W. H. Oswald, Marcos A. Ojeda, Ryan J. Pobanz, Kevin A. Koby, Anthony J. Buchanan, Josh Del Rosso, Mario A. Guzman, and Thomas J. Martin
ACS OMEGA (2021)
https://pubs.acs.org/doi/pdf/10.1021/acsomega.1c04196
Cannabis sativa L. produces over 200 known secondary metabolites that contribute to its distinctive aroma. Studies on compounds traditionally associated with the scent of this plant have focused on those within the terpenoid class. These isoprene-derived compounds are ubiquitous in nature and are the major source of many plant odors. Nonetheless, there is little evidence that they provide the characteristic “skunk-like” aroma of cannabis. To uncover the chemical origins of this scent, we measured the aromatic properties of cannabis flowers and concentrated extracts using comprehensive two-dimensional gas chromatography equipped with time-of-flight mass spectrometry, flame ionization detection, and sulfur chemiluminescence. We discovered a new family of volatile sulfur compounds (VSCs) containing the prenyl (3-methylbut-2-en-1-yl) functional group that is responsible for this scent. In particular, the compound 3- methyl-2-butene-1-thiol was identified as the primary odorant. We then conducted an indoor greenhouse experiment to monitor the evolution of these compounds during the plant’s lifecycle and throughout the curing process. We found that the concentrations of these compounds increase substantially during the last weeks of the flowering stage, reach a maximum during curing, and then drop after just one week of storage. These results shed light on the chemical origins of the characteristic aroma of cannabis and how volatile sulfur compound production evolves during plant growth. Furthermore, the chemical similarity between this new family of VSCs and those found in garlic (allium sativum) suggests an opportunity to also investigate their potential health benefits.
Identification of terpenoid chemotypes among high (?)-trans-?9-tetrahydrocannabinol-producing Cannabis sativa L. cultivars.
Fischedick JT (2017)
Cannabis Cannabinoid Res 2: 34–47
DOI: 10.1089/can.2016.0040
https://www.researchgate.net/publica...va_L_Cultivars
With laws changing around the world regarding the legal status of Cannabis sativa (cannabis) it is important to develop objective classification systems that help explain the chemical variation found among various cultivars. Currently cannabis cultivars are named using obscure and inconsistent nomenclature. Terpenoids, responsible for the aroma of cannabis, are a useful group of compounds for distinguishing cannabis cultivars with similar cannabinoid content. Methods: In this study we analyzed terpenoid content of cannabis samples obtained from a single medical cannabis dispensary in California over the course of a year. Terpenoids were quantified by gas chromatography with flame ionization detection and peak identification was confirmed with gas chromatography mass spectrometry. Quantitative data from 16 major terpenoids were analyzed using hierarchical clustering analysis (HCA), principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and orthogonal partial least squares discriminant analysis (OPLS-DA). Results: A total of 233 samples representing 30 cultivars were used to develop a classification scheme based on quantitative data, HCA, PCA, and OPLS-DA. Initially cultivars were divided into five major groups, which were subdivided into 13 classes based on differences in terpenoid profile. Different classification models were compared with PLS-DA and found to perform best when many representative samples of a particular class were included. Conclusion: A hierarchy of terpenoid chemotypes was observed in the data set. Some cultivars fit into distinct chemotypes, whereas others seemed to represent a continuum of chemotypes. This study has demonstrated an approach to classifying cannabis cultivars based on terpenoid profile.
Not Cannabis specific
Identifying and manipulating structural determinates linking catalytic specificities in terpene synthases.
Greenhagen, B. T., O’Maille, P. E., Noel, J. P., & Chappell, J.
Proceedings of the National Academy of Sciences, 103(26), 9826–9831. (2006).
doi:10.1073/pnas.0601605103
Terpene synthases are a mechanistically intriguing family of enzymes that catalyze complex, multistep reactions that are capable of generating hundreds of structurally diverse hydrocarbon and oxygenated scaffolds of biological and commercial importance. Interestingly, distantly related terpene synthases from fungi to plants all contain an invariant three-dimensional fold, and molecular comparisons of their active sites indicate that they are enriched with relatively inert amino acid residues that do not react directly with the reaction intermediates. Therefore, catalytic specificity appears to rely on the contour and dynamics of the active site created by the positioning of amino acid backbones and side chains on this catalytic surface and by supporting layers of residues surrounding the synthase active site cavity. Despite the high degree of structural relatedness among terpene synthases, previous studies suggest that no clear relationship between phylogenic organization and catalytic specificities is easily deciphered. We now report on the reciprocal interconversion of catalytic specificities between two distinct yet evolutionarily related terpene synthases based on the systematic identification and mutational replacement of variable residues within and surrounding the active site. Furthermore, we uncover previously undocumented biosynthetic activity during the interconversion, activity that could have been present in a common ancestor of these two highly related synthases. These results provide a simplified means for mapping structural features that are responsible for functional attributes and a strategy for identifying residues that differentiate divergent biosynthetic properties in phylogenetically related terpene synthases.
Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils.
Abdollahi, M., Sefidkon, F., Calagari, M., Mousavi, A., & Mahomoodally, M. F.
Industrial Crops and Products, 155, 112793. (2020). doi:10.1016/j.indcrop.2020.112793
Essential oil of Cannabis sativa L. is a valuable bio-product due to its versatility, particularly in terms of its commercial values and potential applications in medicine, cosmetics and bio-pesticide. In this study, the effect of different stages of plant growth on essential oil yield and composition of four hemp varieties, (two monoecious non-native (Fedora 17 and its progeny) and two dioecious native (Fars and Yazd) samples) were investigated. The plant materials, consist of foliage in vegetative stage, inflorescent of flower in flowering stage and in- florescent of seeds in seeding stage were subjected to hydro-distillation. The essential oils were analyzed by GC and GC/MS. The oil yields varied from 0.40 % (Fedora17) to 0.65 % (Yazd). Interaction of cultivar and growth stage showed Fed17?2 at vegetative (0.86 %) and Fed17 at flowering stage (0.20 %), had the most and least oil content, respectively. Twenty-nine compounds were identified representing 81.9%–99.5% of the essential oils. The most abundant sesquiterpenes in the oils were E-caryophyllene (16.40 %–44.70 %), ?-humulene (4.1 %–15.1 %) and Z-caryophyllene (2.4 %–10.7 %), while the major monoterpenes were (0.4–24.9 %), ?-pinene (4.6–24.3 %) and 1,8-cineole (0.8 %–9.3 %) in all growth stages and cultivars. The ratio of sesquiterpenes to monoterpenes were found to decrease during the developing plants. In conclusion, there was no significant difference between mean oil yields of native and non-native samples, but non-native samples produced the highest oil yield in vegetative stage. E-caryophyllene was found at the highest percentage in the oils of nonnative samples at vegetative stage. For all samples, the essential oils at vegetative stage contained much lower production of monoterpenes than flowering stage. In addition, to obtain the highest amount of ?-pinene and 1,8- cineole, the flowering and seeding stages of hemp are recommended.
Impact of Supercritical Fluid Extraction and Traditional Distillation on the Isolation of Aromatic Compounds from Cannabis indica and Cannabis sativa.
Naz, S., Hanif, M. A., Bhatti, H. N., & Ansari, T. M.
Journal of Essential Oil Bearing Plants, 20(1), 175–184.(2017).
doi:10.1080/0972060x.2017.1281766
The chemical composition of essential oils hydrodistilled (HD), steam distilled (SD) and supercritical fluid extracted (SCFE) from the leaves of Cannabis sativa and Cannabis indica from Pakistan were being reported. Maximum yield of essential oil for both strains was obtained at 110°C, 130°C and 45°C for hydrodistillation, steam distillation and supercritical fluid extraction respectively. Yields of essential oil using SCFE technique were more than HD and SD extraction respectively. The main compounds of C. sativa essential oil were characterized by large amounts of caryophyllene (40.6-50.0 %), humulene (9.51-16.0 %), trans-α- bergamotene (4.42-6.31 %) cis-β-farnesene (8.63-9.01 %) and δ-limonene (5.13-8.19 %) respectively. The main components of Cannabis indica were caryophyllene (21.1-25.1 %), carophyllene oxide (4.13-5.02 %), linalool (20.8-22.1 %), trans-α-bergamotene (3.23-5.16 %) and cis-β-farnesene (2.10-3.68 %), menthol (7.20-9.43 %), δ- limonene (6.13-7.19 %), eucalyptol (9.67-12.10 %), and carvone (2.11-5.13 %) respectively.
Improved Profiling of Cannabis Terpenes for Accurate Product Labelling
Laura McGregor and Elinor Hughes
The Column 6 August 2020 Vol 16 Issue 8 Pg 11-16
https://www.sepsolve.com/uploads/bro...20Ad%20Rem.pdf
There are three main sub-species of cannabis—indica, sativa, and ruderalis— but there are hundreds of commercial strains based on these sub-species and their hybrids. Profiling the terpene content in these strains is vital to provide accurate labelling of cannabis-based products, but it can be very challenging. The usual technique for this—one-dimensional gas chromatography (GC)— is not always reliable when it comes to separating the diverse classes of terpenes. This article illustrates how two-dimensional GC (GC ×GC) coupled with mass spectrometry (MS) can be used to profile cannabis terpenes with enhanced separation, resulting in the confident identification of terpenes and improved flavour interpretation.
In Defense of the “Entourage Effect”: Terpenes Found in Cannabis sativa Activate the Cannabinoid Receptor 1 In Vitro
J. LaVigne, Ryan Hecksel, J. Streicher
Published 2020 Biology The FASEB Journal
DOI:10.1096/fasebj.2020.34.s1.04020
Marijuana has been understudied for decades, primarily due to social stigma and legal restrictions. However, as legal restrictions begin to loosen among states, the potential medical benefits and pharmacological properties of marijuana are beginning to be explored. Terpenes, an expansive group of organic chemicals that impart odor and taste, are found in the Cannabis sativa plant and may work synergistically with cannabinoids, such as THC and CBD, in a term deemed the “entourage effect”. Anecdotally among the recreational and medical use community, terpenes have been reported to enhance the potency and physiological effects of marijuana. However, scientific evidence for the “entourage effect” is very limited. To evaluate this hypothesis, we obtained the C. sativa‐relevant terpenes: β‐pinene, α‐humulene, geraniol, and linalool. Utilizing Chinese hamster ovary cells (CHO) expressing the human cannabinoid receptor type 1 (CB1, CB1‐CHO) we screened these terpenes for CB1‐dependent phosphorylation of extracellular signal‐regulated kinase 1/2 (ERK1/2), a well‐known downstream target of CB1 activation, using Western blot. We observed that amp levels were efficaciously stimulated by all four terpenes when compared to positive control, the selective CB1 agonist WIN 55,212‐2. These results appeared to be CB1‐dependent, as pre‐treatment of these cells with a selective CB1 antagonist, rimonabant (SRI141716), blocked ERK phosphorylation by each of the terpenes. We further verified the CB1‐dependent nature of these effects by examining ERK phosphorylation by the terpenes in wild type CHO (WT CHO) cells, which do not express the CB1 receptor. In these cells, β‐pinene and α‐humulene treatment resulted in ERK phosphorylation while linalool or geraniol treatment did not. In WT CHO cells, the ERK phosphorylation induced by β‐pinene and α‐humulene was not CB1‐dependent, as rimonabant pre‐treatment did not block it. These results thus suggest that geraniol and linalool could be CB1‐selective agonists, whereas β‐pinene and α‐humulene are non‐selective and may also activate one or more receptors besides CB1. Follow‐up studies will examine other measures of CB1 activity (binding, amp signaling, GTPgS coupling) to characterize the binding and functional properties of these terpenes at the CB1 receptor, as well as identifying the other targets of β‐pinene and α‐humulene. Once we’ve characterized these terpenes individually, we aim to investigate their role in the “entourage effect”, by testing their modulation of typical cannabinoid (THC, etc.) pharmacology. Translationally, these findings could have implications in marijuana cultivar breeding and could help produce strains optimized for specific terpene profiles, which could be more efficacious for chronic pain management and other therapeutic uses.
Find Pdf
In silico discovery of terpenoid metabolism in Cannabis sativa
Luca Massimino
06 Feb 2017, F1000Res 6:107
doi: 10.12688/f1000research.10778.1
https://www.ncbi.nlm.nih.gov/pmc/art...ch-6-11622.pdf
Due to their efficacy, cannabis based therapies are currently being prescribed for the treatment of many different medical conditions. Interestingly, treatments based on the use of cannabis flowers or their derivatives have been shown to be very effective, while therapies based on drugs containing THC alone lack therapeutic value and lead to increased side effects, likely resulting from the absence of other pivotal entourage compounds found in the Phyto-complex. Among these compounds are terpenoids, which are not produced exclusively by cannabis plants, so other plant species must share many of the enzymes involved in their metabolism. In the present work, 23,630 transcripts from the canSat3 reference transcriptome were scanned for evolutionarily conserved protein domains and annotated in accordance with their predicted molecular functions. A total of 215 evolutionarily conserved genes encoding enzymes presumably involved in terpenoid metabolism are described, together with their expression profiles in different cannabis plant tissues at different developmental stages. The resource presented here will aid future investigations on terpenoid metabolism in Cannabis sativa.
Not Cannabis specific:
Large-Scale Evolutionary Analysis of Genes and Supergene Clusters from Terpenoid Modular Pathways Provides Insights into Metabolic Diversification in Flowering Plants
Johannes A. Hofberger, Aldana M. Ramirez, Erik van den Bergh, Xinguang Zhu, Harro J. Bouwmeester, Robert C. Schuurink, M. Eric Schranz
doi:10.1371/journal.pone.0128808
An important component of plant evolution is the plethora of pathways producing more than 200,000 biochemically diverse specialized metabolites with pharmacological, nutritional and ecological significance. To unravel dynamics underlying metabolic diversification, it is critical to determine lineage-specific gene family expansion in a phylogenomics framework. However, robust functional annotation is often only available for core enzymes catalyzing committed reaction steps within few model systems. In a genome informatics approach, we extracted information from early-draft gene-space assemblies and non-redundant transcriptomes to identify protein families involved in isoprenoid biosynthesis. Isoprenoids comprise terpenoids with various roles in plant-environment interaction, such as pollinator attraction or pathogen defense. Combining lines of evidence provided by synteny, sequence homology and Hidden-Markov-Modelling, we screened 17 genomes including 12 major crops and found evidence for 1,904 proteins associated with terpenoid biosynthesis. Our terpenoid genes set contains evidence for 840 core terpene-synthases and 338 triterpene-specific synthases. We further identified 190 prenyltransferases, 39 isopentenyl-diphosphate isomerases as well as 278 and 219 proteins involved in mevalonate and methylerithrol pathways, respectively. Assessing the impact of gene and genome duplication to lineage-specific terpenoid pathway expansion, we illustrated key events underlying terpenoid metabolic diversification within 250 million years of flowering plant radiation. By quantifying Angiosperm-wide versatility and phylogenetic relationships of pleiotropic gene families in terpenoid modular pathways, our analysis offers significant insight into evolutionary dynamics underlying diversification of plant secondary metabolism. Furthermore, our data provide a blueprint for future efforts to identify and more rapidly clone terpenoid biosynthetic genes from any plant species.
Leaf enclosure measurements for determining volatile organic compound emission capacity from Cannabis spp.
Chi-Tsan Wang, Christine Wiedinmyer, Kirsti Ashworth, William Vizuete Project: Potential Air Quality Impacts of Marijuana Cultivation Facilities in Denver, Colorado October 2018
DOI: 10.1016/j.atmosenv.2018.10.049
The legal commercialization of Cannabis for recreational and medical use in certain US states has effectively created a new and nearly unregulated cultivation industry. Within the city limits of Denver, Colorado, there are now more than 600 registered Cannabis spp. cultivation facilities (CCFs) for recreational and medical uses, each containing thousands of plants. Ambient measurements collected inside growing operations pre-legalization have found concentrations as high as 50–100 ppbv of terpenes; a group of highly reactive biogenic volatile organic compounds (BVOCs) and known precursors for the formation of ozone and particulate matter (PM). Due to its illicit nature there has been insufficient experimental data produced to determine Cannabis spp. emission rates. This study used, for the first time, an enclosure chamber and live Cannabis spp. plants during a 90-day growing period consisting of four different strains of Cannabis spp.: Critical Mass, Lemon Wheel, Elephant Purple, and Rockstar Kush. These measurements enabled characterization of terpenes and estimates of emission capacity (EC, ?gC g?¹ hr?¹) at standard conditions. During peak growth, the percentages of individual BVOC emissions were dominated by ?-myrcene (18–60%), eucalyptol (17–38%), and d-limonene (3–10%) for all strains. Our results showed large variability in the rate and composition of terpene emissions across different strains. For the Critical Mass and Lemon Wheel, the dominant terpenoid was eucalyptol (32% and 38%), and it was ?-myrcene (60% and 45%) for the Elephant Purple and Rockstar Kush. Critical Mass produced the highest terpene emission capacity (8.7 ?gC g?¹ hr?¹) and Rockstar Kush the lowest (4.9 ?gC g?¹ hr?¹). With 600 CCFs in Denver, and assuming 10,000 plants per CCF, an emission capacity of 8.7 ?gC g?¹ hr?¹ would more than double the existing rate of BVOC emissions to 520 metric ton year?¹. Using Maximum Incremental Reactivity (MIR) values the total ozone formation potential from all these emitted species could produce 2100 metric tons year?¹ of ozone, and based on published secondary organic aerosols yields 131 metric tons year?¹ of PM. It is likely that the ECs calculated here are lower than those achieved in CCFs where growing conditions are optimized for rapid growth and higher biomass yields. Further studies including a greater number of the 620 available Cannabis spp. strains and a wider range of treatments are needed to generate a representative dataset. Such a dataset could then better enable assessments of the potential impacts of this new industry on indoor and regional air quality.
Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus
Tarmo Nuutinen
European Journal of Medicinal Chemistry August 2018
DOI: 10.1016/j.ejmech.2018.07.076
Cannabaceae plants Cannabis sativa L. and Humulus lupulus L. are rich in terpenes-both are typically comprised of terpenes as up to 3-5% of the dry-mass of the female inflorescence. Terpenes of cannabis and hops are typically simple mono-and sesquiterpenes derived from two and three isoprene units, respectively. Some terpenes are relatively well known for their potential in biomedicine and have been used in traditional medicine for centuries, while others are yet to be studied in detail. The current, comprehensive review presents terpenes found in cannabis and hops. Terpenes' medicinal properties are supported by numerous in vitro, animal and clinical trials and show anti-inflammatory, antioxidant, analgesic, anticonvulsive, antidepressant, anxiolytic, anticancer, antitumor, neuroprotective, anti-mutagenic, anti-allergic, antibiotic and anti-diabetic attributes, among others. Because of the very low toxicity, these terpenes are already widely used as food additives and in cosmetic products. Thus, they have been proven safe and well-tolerated.
Metabolic Products of Linalool and Modulation of GABAA Receptors
Sinem Milanos, Shaimaa A. Elsharif, Dieter Janzen, Andrea Buettner and Carmen Villmann
Front. Chem., 21 June 2017
DOI: 10.3389/fchem.2017.00046
https://www.frontiersin.org/articles...017.00046/full
Terpenoids are major subcomponents in aroma substances which harbor sedative physiological potential. We have demonstrated that various monoterpenoids such as the acyclic linalool enhance GABAergic currents in an allosteric manner in vitro upon overexpression of inhibitory α1β2 GABAA receptors in various expression systems. However, in plants or humans, i.e., following intake via inhalation or ingestion, linalool undergoes metabolic modifications including oxygenation and acetylation, which may affect the modulatory efficacy of the generated linalool derivatives. Here, we analyzed the modulatory potential of linalool derivatives at α1β2γ2 GABAA receptors upon transient overexpression. Following receptor expression control, electrophysiological recordings in a whole cell configuration were used to determine the chloride influx upon co-application of GABA EC10−30 together with the modulatory substance. Our results show that only oxygenated linalool metabolites at carbon 8 positively affect GABAergic currents whereas derivatives hydroxylated or carboxylated at carbon 8 were rather ineffective. Acetylated linalool derivatives resulted in non-significant changes of GABAergic currents. We can conclude that metabolism of linalool reduces its positive allosteric potential at GABAA receptors compared to the significant potentiation effects of the parent molecule linalool itself.
Metabolism of β-myrcene in vivo and in vitro: its effects on rat-liver microsomal enzymes
K. Madhava Madyastha & V. Srivatsan
XENOBIOTICA, 1987, VOL. 17, NO. 5, 539-549 DOI: 10.3109/00498258709043961
1. Metabolites isolated from the urine of rats after oral administration of /I-myrcene (I) were: 10-hydroxylinalool (11), 7-methyl-3-methylene-oct-6-ene-1,2-diol (IV), 1 - hydroxymethyl-4-isopropenyl cyclohexanol (VI), 10-carboxylinalool (I 11) and 2-hpdroxy7-methyl-3-methylene-oct-6-enoic acid (V).
2. Liver microsomes prepared from phenobarbital-treated rats convert β-myrcene (I) to 10-hydroxylinalool (II) in the presence of NADPH and oxygen. NADH neither supported this reaction nor did it show any synergistic effect. The rate of conversion was significantly greater in microsomes prepared from phenobarbital-treated rats than from 3-methylcholanthrene-treated or control microsomal preparations. The formation of 10-hydroxylinalool (II) was inhibited by metyrapone, carbon monoxide, SKF-525A, p-chloromercuric benzoate (p-CMB) and cytochrome c.
3. Titration of phenobarbital-induced liver microsomes with β-myrcene (I) produced a series of type I difference spectra with peaks around 387–390 nm and troughs around 421–425 nm. The Ks for β-myrcene was 10.6 μM.
4. Administration (four days) of β-myrcene (I) to rats did not result in any significant effect on the hepatic drug-metabolizing enzymes.
Metabolomics and bioanalysis of terpenoid derived secondary metabolites: Analysis of Cannabis sativa L. metabolite production and prenylases for cannabinoid production.
Muntendam, R. (2015). PHD Thesis
https://www.rug.nl/research/portal/f...ete_thesis.pdf
The research described within this thesis is focused on the analysis of cannabinoid production patterns exhibited by selected strains from the medicinal Cannabis cultivator Bedrocan (Bedrocan B.V., Veendam). Furthermore, the enzymatic production of cannabigerolic acid is explored by analyzing the catalytic mechanism of a prenyltransferase. At the end the putative terpene based production host Xanthophyllomyces dendrorhous is tested for metabolic engineering purposes by the introduction of a terpenoid cyclase to serve as proof of principle and to obtain molecular strategies to genetically alter the host for non-native compound production, such as cannabinoids
Modelling of a limonene/pinene synthase and product specifity of the two monoterpene synthases of Cannabis sativa L
Brandt W, Weber R, Gu?nnewich N, Bräuer L, Schulze D, Rausch F, Page JE,
Schmidt J, Kutchan TM, Wessjohann LA. (2008)
Find DOI or Link I asked Dr Gu?nnewich where to find it.
MONO- AND SESQUI-TERPENE HYDROCARBONS OF THE ESSENTIAL OIL OF CANNABIS SATIVA
HENK HENDRIKS, THEO M. MALINGR, SIEB BATTERMAN, REIN BOS
Phytochemistry, 1975, Vol. 14. Pp 814-815
FIND DOI or Link
The presence of cannabinoids in the essential oil of Cannabis sativu L. has been reported previously In this communication the results of the investigation into the mono- and sesqui-terpenc hydrocarbon fraction are presented. together with previous work (Table I).
Monocyclic and bicyclic monoterpenes in air of German daycare centers and human biomonitoring in visiting children, the LUPE 3 study Lukas Schmidt, Thomas Lahrz, Martin Kraft, Thomas Göen, Hermann Fromme
Environment International 83 (2015) 86–9
DOI: 10.1016/j.envint.2015.06.004
To investigate the assumed association between indoor air pollution with monoterpenes (MTps) and the internal MTp exposure of occupants, a comparative study was performed in daycare centers in two federal states of Germany. Three well-known monoterpenoid air pollutants, viz. α-pinene (αPN), Δ3 -carene (CRN), and R-limonene (LMN), were measured in indoor air in 45 daycare centers. Additionally, urine samples of 222 children visiting these facilities were collected in the evening after a full-day stay. Altogether 11 MTp metabolites were analyzed in the urine samples using a novel highly sensitive and selective gas chromatographic–tandemmass spectrometric procedure. The medians (95th percentiles) of the MTp levels in indoor air were 9.1 μg m−3 (94 μg m−3 ) for LMN, 2.6 μg m−3 (13 μg m−3 ) for αPN, and b1.0 μg m−3 (3.2 μg m−3 ) for CRN. None of the day care centers exceeded the German health precaution or hazard guide value. In spite of the low MTp air exposure, the urine analyses revealed an exposure to the three monoterpenes in almost all children. The median levels of MTp metabolites in urine were 0.11 mg L−1 for LMN-8,9-OH, 0.10 mg L−1 for LMN-1,2- OH, 49 μg L−1 for PA, 2.9 μg L−1 for POH, 5.2 μg L−1 for tCAR, and 4.1 μg L−1 for cCAR (LMN metabolites), 7.2 μg L−1 for MYR, 19 μg L−1 for tVER, and 19 μg L−1 for cVER (αPN metabolites), as well as 8.2 μg L−1 for CRN-10-COOH (CRN metabolite). Statistically significant and strong correlations among the urinary metabolites of each MTp were found. Moreover, statistical associations between LMN metabolites and the LMN indoor air levels were revealed. However, the weakness of the associations indicates a considerable impact of other MTp sources, e.g. diet and consumer products, on the internal exposure
Not Cannabis Specific
Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants.
Degenhardt, J., Köllner, T. G., & Gershenzon,
J. Phytochemistry, 70(15-16), 1621–1637. (2009).
doi:10.1016/j.phytochem.2009.07.030
The multitude of terpene carbon skeletons in plants is formed by enzymes known as terpene synthases. This review covers the monoterpene and sesquiterpene synthases presenting an up-to-date list of enzymes reported and evidence for their ability to form multiple products. The reaction mechanisms of these enzyme classes are described, and information on how terpene synthase proteins mediate catalysis is summarized. Correlations between specific amino acid motifs and terpene synthase function are described, including an analysis of the relationships between active site sequence and cyclization type and a discussion of whether specific protein features might facilitate multiple product formation
Not Cannabis specific
More is better: the diversity of terpene metabolism in plants.
Zhou, F., & Pichersky, E.
Current Opinion in Plant Biology, 55, 1–10.(2020).
doi:10.1016/j.pbi.2020.01.005
All plants synthesize a diverse array of terpenoid metabolites. Some are common to all, but many are synthesized only in specific taxa and presumably evolved as adaptations to specific ecological conditions. While the basic terpenoid biosynthetic pathways are common in all plants, recent discoveries have revealed many variations in the way plants synthesized specific terpenes. A major theme is the much greater number of substrates that can be used by enzymes belonging to the terpene synthase (TPS) family. Other recent discoveries include non-TPS enzymes that catalyze the formation of terpenes, and novel transport mechanisms.
[FONT=PÊˇø◊îúY¿¥*†°∂‡XËÊˇø0IπY¥ü]More Than Just Taste and Smell: Examining the Complexity and Diversity of Terpene Profiles from Popular Cannabis Strains
Andrew Defries
[FONT=PÊˇø◊îúY¿¥*†°∂‡XËÊˇø0IπY¥ü]https://thecannabinoidchronicles.com/author/andrew/[/FONT]
[FONT=PÊˇø◊îúY¿¥*†°∂‡XËÊˇø0IπY¥ü]https://terpenecharts.com/author/andrew/[/FONT]
[FONT=PÊˇø◊îúY¿¥*†°∂‡XËÊˇø0IπY¥ü]https://cannabinoidcharts.com/author/andrew/[/FONT][/FONT]
[FONT=PÊˇø◊îúY¿¥*†°∂‡XËÊˇø0IπY¥ü]Poster · April 2018
[FONT=PÊˇø◊îúY¿¥*†°∂‡XËÊˇø0IπY¥ü]https://www.researchgate.net/publication/325270252_More_Than_Just_Taste _and_Smell_Examining_the_Compl exity_and_Diversity_of_Terpene _Profiles_from_Popular_Cannabi s_Strains?enrichId=rgreq-39ad91745ca344fd0905646f59c829 f3-XXX&enrichSource=Y292ZXJQYWdlO zMyNTI3MDI1MjtBUzo2OTA5OTMxNzk 0MDIyNDdAMTU0MTc1NzAyMDk1Mw%3D %3D&el=1_x_3&_esc=publicationC overPdf[/FONT] [/FONT]
Cannabis synthesizes a diversity of secondary metabolites. Selection and breeding has resulted in strains with enhanced cannabinoid potency and unique flavor profiles called chemotypes. Volatile organic compounds known as terpenes responsible for the smell and taste of cannabis are becoming recognized as medicinal molecules in their own right. Our goal is to create a botanical classification system to delineate chemotype groups and subgroups in popular strains using terpene profiles. When used in conjunction with genetic data we aim to determine the lineage of unknown strains and to guide future breeding projects to develop specific and unique chemotypes.
Multidimensional analysis of cannabis volatile constituents: Identification of 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane as a volatile marker of hashish, the resin of Cannabis sativa L.
Marie Marchinia, Céline Charvozb, Laurence Dujourdyb, Nicolas Baldovinia, Jean-Jacques Filippia, ,
Journal of Chromatography Volume 1370, 28 November 2014, Pages 200–215
Doi: 10.1016/j.chroma.2014.10.045
•Analysis of cannabis herb and hashish volatile constituents by HS-SPME-GC × GC–MS.
•Identification of a new volatile marker of hashish.
•Photolytic rearrangement of ?-myrcene into hashishene.
•Formation of photo-oxidation products during hashish manufacture.
The volatile constituents of drug samples derived from Cannabis sativa L. were investigated by means of headspace solid phase microextraction (HS-SPME) and gas chromatography techniques (GC–MS, GC × GC–MS). Samples of cannabis herb andhashish showed clear differences in their volatile chemical profiles, mostly resulting from photo-oxidation processes occurring during the transformation of fresh cannabis herb into hashish. Most unexpectedly, we could demonstrate hashish samples as containing remarkable amounts of a rare and unusual monoterpene – 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane – among the volatile compounds detected in their headspaces. We gave evidence for the formation of this compound from the light induced rearrangement of ?-myrcene during the manufacture of hashish. In view of its high abundance among volatile constituents of cannabis resin and its scarce occurrence in other natural volatile extracts, we propose to rename this specific monoterpene hashishene.
Natural Borneol, a Monoterpenoid Compound, Potentiates Selenocystine-Induced Apoptosis in Human Hepatocellular Carcinoma Cells by Enhancement of Cellular Uptake and Activation of ROS-Mediated DNA Damage.
Su, J., Lai, H., Chen, J., Li, L., Wong, Y.-S., Chen, T., & Li, X. (2013).
PLoS ONE, 8(5), e63502.
doi:10.1371/journal.pone.0063502
Selenocystine (SeC) has been identified as a novel compound with broad-spectrum anticancer activities. Natural borneol (NB) is a monoterpenoid compound that has been used as a promoter of drug absorption. In the present study, we demonstrated that NB significantly enhanced the cellular uptake of SeC and potentiated its antiproliferative activity on HepG2 cells by induction of apoptosis. NB effectively synergized with SeC to reduce cancer cell growth through the triggering apoptotic cell death. Further mechanistic studies by Western blotting showed that treatment of the cells with NB and SeC activated the intrinsic apoptotic pathway by regulation of pro-survival and pro-apoptotic Bcl-2 family proteins. Treatment of the cells with NB and SeC induced the activation of p38MAPK and inactivation of Akt and ERK. NB also potentiated SeC to trigger intracellular ROS generation and DNA strand breaks as examined by Comet assay. Moreover, the thiol-reducing antioxidants effectively blocked the occurrence of cell apoptosis, which confirmed the important role of ROS in cell apoptosis. Taken together, these results reveal that NB strongly potentiates SeC-induced apoptosis in cancer cells by enhancement of cellular uptake and activation of ROS-mediated DNA damage. NB could be further developed as a chemosensitizer of SeC in treatment of human cancers.
Not Cannabis specific
Natural Terpenes Prevent Mitochondrial Dysfunction, Oxidative Stress and Release of Apoptotic Proteins during Nimesulide-Hepatotoxicity in Rats
Brijesh Kumar Singh1, Madhulika Tripathi, Bhushan P. Chaudhari, Pramod K. Pandey, Poonam Kakkar
PLoS ONE 7(4): e34200.
doi:10.1371/journal.pone.0034200
Nimesulide, an anti-inflammatory and analgesic drug, is reported to cause severe hepatotoxicity. In this study, molecular mechanisms involved in deranged oxidant-antioxidant homeostasis and mitochondrial dysfunction during nimesulide induced hepatotoxicity and its attenuation by plant derived terpenes, camphene and geraniol has been explored in male Sprague-Dawley rats. Hepatotoxicity due to nimesulide (80 mg/kg BW) was evident from elevated SGPT, SGOT, bilirubin and histo-pathological changes. Antioxidants and key redox enzymes (iNOS, mtNOS, Cu/Zn-SOD, Mn-SOD, GPx and GR) were altered significantly as assessed by their mRNA expression, Immunoblot analysis and enzyme activities. Redox imbalance along with oxidative stress was evident from decreased NAD(P)H and GSH (56% and 74% respectively; P,0.001), increased superoxide and secondary ROS/RNS generation along with oxidative damage to cellular macromolecules. Nimesulide reduced mitochondrial activity, depolarized mitochondria and caused membrane permeability transition (MPT) followed by release of apoptotic proteins (AIF; apoptosis inducing factor, EndoG; endonuclease G, and Cyto c; cytochrome c). It also significantly activated caspase-9 and caspase-3 and increased oxidative DNA damage (level of 8-Oxoguanine glycosylase; P,0.05). A combination of camphene and geraniol (CG; 1:1), when pre-administered in rats (10 mg/kg BW), accorded protection against nimesulide hepatotoxicity in vivo, as evident from normalized serum biomarkers and histopathology. mRNA expression and activity of key antioxidant and redox enzymes along with oxidative stress were also normalized due to CG pre-treatment. Downstream effects like decreased mitochondrial swelling, inhibition in release of apoptotic proteins, prevention of mitochondrial depolarization along with reduction in oxidized NAD(P)H and increased mitochondrial electron flow further supported protective action of selected terpenes against nimesulide toxicity. Therefore CG, a combination of natural terpenes prevented nimesulide induced cellular damage and ensuing hepatotoxicity
Odor Scrubbing in a Growhouse: Ways to Remove Odor from Cannabis Terpenes
Antonio DeRose
Terpenes And Testing Magazine
https://terpenesandtesting.com/odor-...abis-terpenes/
The smell of cannabis comes from its terpene profile. Terpenes have very distinct and often strong odors, and the collective odor of a given plant is one way many consumers choose which types of flower they want to consume. The strong aromas from terpenes may be well-liked by many consumers, but odor is an ongoing concern for cannabis grow facilities that must follow local city and state regulations regarding odor output. For this reason, many facilities utilize different odor scrubbing techniques to reduce and eliminate the strong odors of the cannabis being cultivated.
Opioids Out, Cannabis In Negotiating the Unknowns in Patient Care for Chronic Pain
JAMA November 1, 2016 Volume 316, Number 17 Pg 1763-4
DOI: 10.1001/jama.2016.13677
With thecurrent nationwide epidemicof opioidabuse, dependence, and fatalities, clinicians are being asked by federal agencies and professional societies to control their prescribing of narcotic medications for pain. Federal guidelines emphasize tapering, discontinuing, and limiting initiation of these drugs except in provision of end of-life care.1 Reducing reliance on opioids, however, is a
massive task. According to one estimate, more than 650 000 opioid prescriptions are dispensed each day in the United States. Unless the nation develops an increased tolerance to chronic pain, reduction in opioid prescribing leaves a vacuum that will be filled with other therapies
para-Menthane as a Stable Terpene Derived from Orange By-Products as a Novel Solvent for Green Extraction and Solubilization of Natural Substances.
Madji, Hilali, Fabiano-Tixier, Tenon, Bily, Laguerre, & Chemat.
Molecules, 24(11), 2170.(2019).
doi:10.3390/molecules24112170
This study aims at investigating p-menthane, a novel bio-based solvent resulting from the hydrogenation of d-limonene, as a green alternative to n-hexane or toluene for the extraction and solubilization of natural substances. First, conductor-like combination of quantum chemistry (COSMO) coupled with statistical thermodynamics (RS) calculations show a comparable solubilization profile of p-menthane and n-hexane for carotene, volatile monoterpenes such as carvone and limonene, and model triglycerides. Other data obtained experimentally in solid/liquid extraction conditions further indicate that p-menthane showed similar performances to n-hexane for extracting carotenes from carrots, aromas from caraway seeds, and oils from rapeseeds, as these products showed a comparable composition. p-Menthane was also tested using common analytical extraction procedures such as Soxhlet for determination of oil content via multiple extraction stages, and Dean–Stark for determination of water content via azeotropic distillation. For both systems, yields were comparable, but for Dean–Stark, the distillation curve slope was higher when using p-menthane, and the time needed to attain 100% water recovery was 55% shorter than for toluene. Taken together, these results reveal the potential of p-menthane as a green replacer for petroleum-based solvents such as n-hexane or toluene.
Phytol, not propylene glycol, causes severe pulmonary injury after inhalation dosing in Sprague-Dawley rats
Daniela Schwotzer, Andrew Gigliotti, Hammad Irshad, Wendy Dye & Jacob McDonald
Inhalation Toxicology 2021, VOL. 33, NO. 1, 33–40
DOI: 10.1080/08958378.2020.1867260
Introduction: The use of vaping pens for inhalation of cannabinoid derived products is rising and has become a popular alternative to smoking combustible products. For efficient product delivery, additives are sometimes added and vaping pens often may include compounds like Phytol or Propylene
Glycol as thinning agents. This study aimed at comparing Phytol and Propylene Glycol with respect to potential toxicity and safe use in vaping products.
Methods: Male and female Sprague Dawley rats were exposed to 5mg/L of Phytol or Propylene Glycol for up to 6 hours over up to 14 days and monitored for clinical signs and changes in body weight. Gross necropsy and histopathology of respiratory tissue was performed to assess potential adverse effects.
Results: Phytol exposed animals expressed severe clinical signs, body weight loss and mortality after one or two exposure days, leading to termination of all dose groups for this compound. Lung weights were increased and respiratory tissue was severely affected, demonstrating dose-responsive tissue degeneration, necrosis, edema, hemorrhage and inflammation. Propylene Glycol exposed animals did not show any adverse reactions after 14 days of high dose exposure.
Conclusions: For Phytol, a low observed adverse effect level (LOAEL) was determined at _109.0/ 10.9 mg/kg/day presented/deposited dose and therefore its use as excipient in vaping product is not recommend; a safe exposure range was not established for Phytol. Propylene Glycol, in contrast, is considered safe with a no observed adverse effect level (NOAEL) at 1151.7/115.2mg/kg/day presented/ deposited dose in rats.
Not Cannabis specific
Plant terpenoid synthases: Molecular biology and phylogenetic analysis
JO¨RG BOHLMANN, GILBERT MEYER-GAUEN AND RODNEY CROTEAU
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 4126–4133, April 1998
doi: 10.1073/pnas.95.8.4126
https://www.pnas.org/content/pnas/95/8/4126.full.pdf
This review focuses on the monoterpene, sesquiterpene, and diterpene synthases of plant origin that use the corresponding C10, C15, and C20 prenyl diphosphates as substrates to generate the enormous diversity of carbon skeletons characteristic of the terpenoid family of natural products. A description of the enzymology and mechanism of terpenoid cyclization is followed by a discussion of molecular cloning and heterologous expression of terpenoid synthases. Sequence relatedness and phylogenetic reconstruction, based on 33 members of the Tps gene family, are delineated, and comparison of important structural features of these enzymes is provided. The review concludes with an overview of the organization and regulation of terpenoid metabolism, and of the biotechnological applications of terpenoid synthase genes.
Plucking Terpenes from Plants
Jason S. Lupoi
https://extractionmagazine.com/2020/...zHZA8S0Yq2A_wQ
Three ways for extracting fragrant botanical essences
Steam Distillation
Subcritical CO2
Hexane
Potentiating effect of ?-caryophyllene on anticancer activity of ?-humulene, isocaryophyllene and paclitaxel.
Legault, J., & Pichette, A.
Journal of Pharmacy and Pharmacology, 59(12), 1643–1647 (2007)
doi:10.1211/jpp.59.12.0005
b-caryophyllene is a sesquiterpene widely distributed in essential oils of various plants. Several biological activities are attributed to b-caryophyllene, such as anti-inflammatory, antibiotic, antioxidant, anticarcinogenic and local anaesthetic activities. In this work, the potentiating effect of bcaryophyllene on the anticancer activity of a-humulene, isocaryophyllene and paclitaxel against MCF-7, DLD-1 and L-929 human tumour cell lines was evaluated. A non-cytotoxic concentration of bcaryophyllene significantly increased the anticancer activity of a-humulene and isocaryophyllene on MCF-7 cells: a-humulene or isocaryophyllene alone (32 mg mL?1) inhibited cell growth by about 50% and 69%, respectively, compared with 75% and 90% when combined with 10 mg mL?1 b-caryophyllene. Moreover, b-caryophyllene potentiated the anticancer activity of paclitaxel on MCF-7, DLD-1 and L-929 cell lines. The highest potentiating effect was obtained in DLD-1 cells treated with paclitaxel combined with 10 mg mL?1 b-caryophyllene, which increased the paclitaxel activity about 10-fold. The intracellular accumulation of paclitaxel-oregon green was evaluated in combination with concentrations of b-caryophyllene ranging from 2.5 to 40 mg mL?1. b-Caryophyllene (10 mg mL?1) significantly increased the intracellular accumulation of paclitaxel-oregon green (about 64% over controls). Moreover, b-caryophyllene induced intracellular accumulation of calcein but not verapamil, an inhibitor of P-glycoprotein and multidrug resistance related protein transporters, suggesting that b-caryophyllene promotes drug accumulation by a different mechanism of action. These results
suggest that b-caryophyllene facilitates the passage of paclitaxel through the membrane and thus potentiates its anticancer activity.
Predicting chemovar cluster and variety verification in vegetative cannabis accessions using targeted single nucleotide polymorphisms
Philippe Henry, Aaron Hilyard , Steve Johnson , Cindy Orser
PeerJ Preprints (2018)
DOI: 10.7287/peerj.preprints.27442v1
The cannabis industry has gained momentum and global acceptance recently, culminating in the legalization of adult use at the federal level in Canada, a first among G20 countries. Inherent to legalization, a highly regulated regime has emerged, mostly centered on end user safety, restriction of access to youth, and diversion of market shares away from the black market and organized crime. The lack of authentication of cannabis varieties remains as an issue often unaddressed by the regulators, although this has the potential to seriously hamper research and the medical application of cannabis derived products. Here, we extend upon previous work that aims to classify cannabis accessions based on their dominant terpene profiles, focusing on four main informative terpenes, betamyrcene, terpinolene, limonene and beta-caryophyllene. We identify three major terpene groups and present a simple genetic-based tool to bridge the variety identification gap and to enable the prediction of terpenoid expression in vegetative cannabis. This genetic tool offers promise to sorting out the strain name game that has been ongoing, thus providing greater transparency in the industry and contributing to an enhanced understanding of cannabis medicine for the end user
Not Cannabis related
Prevalence of cilantro (Coriandrum sativum) disliking among different ethnocultural groups.
Mauer, L., & El-Sohemy, A.
Flavour, 1(1), 8. (2012).
doi:10.1186/2044-7248-1-8
Background: Cilantro, the leaf of the Coriandrum sativum plant, is an herb that is widely consumed globally and has purported health benefits ranging from antibacterial to anticancer activities. Some individuals report an extreme dislike for cilantro, and this may explain the different cilantro consumption habits between populations. However, the prevalence of cilantro dislike has not previously been reported in any population. The objective of this study was to determine the prevalence of cilantro dislike among different ethnocultural groups from a population of young adults living in Canada. Subjects (n = 1,639) between the ages of 20 and 29 years were participants of the Toronto Nutrigenomics and Health Study. Individuals rated their preference for cilantro on a 9-point scale from ‘dislike extremely’ to ‘like extremely’. Subjects also had the option to select ‘have not tried’ or ‘would not try’. Subjects who selected 1 to 4 were classified as disliking cilantro. Results: The prevalence of dislike ranged from 3 to 21%. The proportion of subjects classified as disliking cilantro was 21% for East Asians, 17% for Caucasians, 14% for those of African descent, 7% for South Asians, 4% for Hispanics, and 3% for Middle Eastern subjects. Conclusions: These findings show that the prevalence of cilantro dislike differs widely between various ethnocultural groups.
Quantitative Determination of Terpenes in Cannabis Using Headspace Solid Phase Microextraction and GC/MS
Michael Halpenny, Katherine K. Stenerson
GERSTEL Application Note No. 189 , 2017
https://www.gerstel.com/pdf/AppNote-189.pdf
Well known for their characteristic fl avor and fragrance characteristics, terpenes are contained in the derived essential oils of cannabis. Analysis of cannabis for terpene concentrations can be applied to strain identifi cation, referred to as fi ngerprinting, and for concentration accuracy when applied to medicinal treatments. Terpenes have high vapor pressures, are extremely volatile and thus are an excellent candidate for static headspace GC analysis. In this work, headspace SPME (HS-SPME) was combined with GC/ MS for the quantitative analysis of several selected terpenes in cannabis. The conventional approach for terpene analysis in cannabis involves a solvent extraction followed by GC/FID analysis. HS-SPME offers several advantages over the solvent extraction method in that it is non-destructive to the sample, requires a very small sample size, produces minimal interference from co-extracted matrix, protects the GC instrument from contamination, and can be easily automated
Qualitative terpene profiling of Cannabis varieties cultivated for medical purposes
Ernesto D Rocha, Vitória EA Silva, Fernanda CS Pereira, Valery M Jean, Fabio L Costa Souza, Leopoldo Clemente Baratto, Ana Vieira, Virginia M Carvalho
Rodriguesia 71January 2020
DOI: 10.1590/2175-7860202071040
https://www.researchgate.net/publica...dical_purposes
https://www.scielo.br/j/rod/a/k69ddv...BGqmS/?lang=en
With the upcoming medical Cannabis regulation, quality control methods on raw material will be required. Besides testing for contaminants and potency, there are also pharmaceutical and forensic interests in the determination of the terpene profile in different strains of Cannabis as complementary identification methods. A simple non-destructive HS-SPME GC-MS method was used to identify the terpene content in twelve Cannabis samples, four of them were of the hemp type (Harle-tsu), seven from various marihuana types and one of the intermediate type. They all were previously analyzed by HPLC to determine the potency (THC and CBD content). Spectral library matching was used to identify the terpenes compounds. Thirty terpenes compounds were detected, nine of them were present in all Cannabis samples and used to find their terpene profile: α-pinene, β-pinene, β-myrcene, D-limonene, terpinolene, linalool, caryophyllene, α-bergamotene and humulene. Three of them, caryophyllene, α-pinene and β-myrcene were found as larger components in most of samples. A principal components analyses (PCA) was performed. The four hemp type samples showed two different profiles, two samples showed caryophyllene as main component and the others two with β-myrcene as such. The marihuana type samples showed wider profiles with no clear patterns at all, which is not surprising because of the low number of samples. The simple methodology shows viable to set the terpenes profile for analyses of raw Cannabis material. Suitability for differentiation between different sorts of types needs more studies, with increasing numbers of samples.
Quantitation of Select Terpenes/Terpenoids and Nicotine Using Gas Chromatography−Mass Spectrometry with High-Temperature Headspace Sampling
Trinh-Don Nguyen, Seamus Riordan-Short, Thu-Thuy T. Dang, Rob O’Brien, and Matthew Noestheden
ACS Omega 2020, 5, 5565−5573
DOI:10.1021/acsomega.0c00384
https://www.ncbi.nlm.nih.gov/pmc/art.../ao0c00384.pdf
Plants are the main sources of many high-value bioactive terpenoids used in the medical, fragrance, and food industries. Increasing demand for these bioactive plants and their derivative products (e.g., cannabis and extracts thereof) requires robust approaches to verify feedstock, identify product adulteration, and ensure product safety. Reported here are singlelaboratory validation details for a robust testing method to quantitate select terpenes and terpenoids in dry plant materials and terpenoid-containing vaping liquids (e.g., a derivative product) using high-temperature headspace gas chromatography−mass spectrometry, with glycerol used as a headspace solvent. Validated method recoveries were 75−103%, with excellent repeatability (relative standard deviation (RSD) < 5%) and intermediate precision (RSD < 12%). The use of high-temperature headspace (180 °C) permitted terpene and terpenoid profiles to be monitored at temperatures consistent with vaping conditions.
Quantitation of Terpenes in Cannabis Products Using APCI LC-MS/MS
Katherine C Hyland, C Borton, P Winkler, Matthew Noestheden
Planta Medica 82(05) March 2016
DOI: 10.1055/s-0036-1579770
With the recent legalization of cannabis in several states, there is a growing need for robust, cost-effective analytical methods to facilitate routine testing. While testing of potency (and for pesticide and herbicide residues) is important, manufacturers of cannabis products also need fit-to-purpose analytical methods that provide information on the sensory profile of their products to ensure lot-to-lot consistency. Here, we present an LCMS/MS method that uses atmospheric pressure chemical ionization (APCI) for the analysis of terpenes (major determinant of aroma) in cannabis products. Examples of accurate quantitation are shown for a variety of cannabis products.
R Limonene metabolism in humans and metabolite kinetics after oral administration
Lukas Schmidt · Thomas Göen
Arch Toxicol
DOI 10.1007/s00204-016-1751-6
We studied the R-limonene (LMN) metabolism and elimination kinetics in a human in vivo study. Four volunteers were orally exposed to a single LMN dose of 100–130 µg kg−1 bw. In each case, one pre-exposure and subsequently all 24 h post-exposure urine samples were collected. From two subjects, blood samples were drawn up to 5 h after exposure. The parent compound was analysed in blood using headspace GC–MS. The metabolites cis- and trans-carveol (cCAR), perillyl alcohol (POH), perillic acid (PA), limonene-1,2-diol (LMN-1,2-OH), and limonene-8,9-diol (LMN-8,9-OH) were quantified in both blood and urine using GC-PCI-MS/MS. Moreover, GC-PCI-MS full-scan experiments were applied for identification of unknown metabolites in urine. In both matrices, metabolites reached maximum concentrations 1–2 h post-exposure followed by rapid elimination with half-lives of 0.7–2.5 h. In relation to the other metabolites, LMN-1,2-OH was eliminated slowest. Nonetheless, overall renal metabolite elimination was completed within the 24-h observation period. The metabolite amounts excreted via urine corresponded to 0.2 % (cCAR), 0.2 % (tCAR), <0.1 % (POH), 2.0 % (PA), 4.3 % (LMN-1,2-OH), and 32 % (LMN-8,9-OH) of the orally administered dose. GC-PCI-MS full-scan analyses revealed dihydroperillic acid (DHPA) as an additional LMN metabolite. DHPA was estimated to account for 5 % of the orally administered dose. The study revealed that human LMN metabolism proceeds fast and is characterised by oxidation mainly of the
exo-cyclic double bond but also of the endo-cyclic double bond and of the methyl side chain. The study results may support the prediction of the metabolism of other terpenes or comparable chemical structures.
Removal of floral microbiota reduces floral terpene emissions
Josep Penuelas, Gerard Farre-Armengol, Joan Llusia, Albert Gargallo-Garriga, Laura Rico, Jordi Sardans, Jaume Terradas & Iolanda Filella.
DOI: 10.1038/srep06727
The emission of floral terpenes plays a key role in pollination in many plant species. We hypothesized that the floral phyllospheric microbiota could significantly influence these floral terpene emissions because microorganisms also produce and emit terpenes. We tested this hypothesis by analyzing the effect of removing the microbiota from flowers. We fumigated Sambucus nigra L. plants, including their flowers, with a combination of three broad-spectrum antibiotics and measured the floral emissions and tissular concentrations in both antibiotic-fumigated and non-fumigated plants. Floral terpene emissions decreased by ca. two thirds after fumigation. The concentration of terpenes in floral tissues did not decrease, and floral respiration rates did not change, indicating an absence of damage to the floral tissues. The suppression of the phyllospheric microbial communities also changed the composition and proportion of terpenes in the volatile blend. One week after fumigation, the flowers were not emitting b-ocimene, linalool, epoxylinalool, and linalool oxide. These results show a key role of the floral phyllospheric microbiota in the quantity and quality of floral terpene emissions and therefore a possible key role in pollination.
Selective recovery of terpenes, polyphenols and cannabinoids from Cannabis sativa L. inflorescences under microwaves Veronika Gunjević, Giorgio Grillo, Diego Carnaroglio, Arianna Binello, Alessandro Barge, Giancarlo Cravotto
Industrial Crops and Products Volume 162, April 2021 DOI: 10.1016/j.indcrop.2021.113247
In recent years, hemps health and nutritional properties recognition has led to an impressive growth of Cannabis research, industrial processing, and the related market. Moreover, the demand for natural Cannabis-derived compounds (i.e. terpenes, polyphenols, and cannabinoids) is constantly growing. In spite of the strict regulation of some countries, the global market needs suitable technologies for the smart recovery of bioactive Cannabis metabolites. Conventional extraction procedures can show drawbacks, in terms of environmental impact and their high energy consumption. Microwaves (MW), a mature technique for extraction-process intensification, is attracting great amounts of attention in academic-research and industrial-application fields for its technological advantages. This work aims to design a fast and cost-efficient MW-assisted cascade protocol for bioactive Cannabis compounds recovery in a pilot-scale reactor. Microwave-assisted hydrodistillation (MAHD) can provide a volatile hydrodistillate that is rich in monoterpenes, sesquiterpenes, and a small amount of phytocannabinoids. Using non-canonical protocol of hydrodistillation, the definition of “volatile fraction” is generally considered more appropriate than “essential oil”. The health-promoting activity of this combination has been proposed in literature, and can constitute matter of further investigations. The optimized MAHD procedure yielded 0.35 ± 0.02 % w/w of hydrodistillate, while conventional hydrodistillation gave only 0.12 ± 0.01 %, w/w (in relation to dry inflorescence mass). The water resulting in the vessel after MAHD showed a high total polyphenolic content (5.35 ± 0.23 %, w/w). Two flavones known for their beneficial effects to health, namely luteolin-7-O-glucoside and apigenin-7-O-glucoside, were detected and quantified. An attempt to recover phytocannabinoid using the MW-assisted hydrodiffusion and gravity method (MAHG) was also carried out. Cannabinoids (CBD and THC) content was determined in fresh Cannabis and in production streams. During MAHD, phytocannabinoid decarboxylation inside the residual matrix was around 70 % (69.01 ± 0.98 % and 74.32 ± 1.02 % for THC and CBD respectively). Furthermore, the overall content of these metabolites was not affected by the hydrodistillation, preserving the processed plant material for subsequent ethanolic extraction.
Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation
Carla Da Porto, Deborha Decorti, Andrea Natolino
Industrial Crops and Products 58 (2014) 99–103
doi.org/10.1016/j.indcrop.2014.03.042
The use of supercritical carbon dioxide (Sc-CO2) extraction at 10 and 14 MPa and 40?C and on-line frac-tionation using two separators (Sep 1: 7 MPa/25?C; Sep2: 5 MPa/15?C) to recovery volatile compoundsfrom the inflorescences of fiber type Cannabis sativa L. was investigated by HS-SPME/GC–MS and direct GC–MS and compared with hydrodistillation. The best results were obtained by Sc-CO2extraction car-ried out at 10 MPa and 40?C. Under these operating conditions, cuticular waxes covering the surface offlowers were collected in the first separator and volatile compounds (100%) in the second. The superiorquality of this last extract was proved by the perfect overlapping of its HS-SPME/GC–MS volatile profileto that of inflorescences. The recovery of fractions with different composition and biological properties,made the inflorescences of fiber type Cannabis sativa L suitable for cosmetic and/or food industry.
Simplified Cannabis Terpene Profiling by GCMS
SHIMADZU
First Edition: September 2016
https://www.shimadzu.eu.com/sites/sh...ngcannabis.pdf
Terpene and terpenoid compounds are naturally occurring aromatic compounds that give cannabis its unique flavor and fragrance. Aside from their aromatic properties, terpenes have advantageous health benefits. They also have a synergistic relationship with cannabinoids, which further enhance the therapeutic effect of THC. Cannabis has over 140 terpene components, many of which are of medicinal interest. 1 Predominant terpenes in cannabis include β-myrcene, which has antibiotic properties and enhances the THC muscle relaxant effect; α-pinene, which has antiinflammatory properties and enhances the THC bronchodilator effect; and β-caryophyllene, which also has anti-inflammatory properties and enhances the THC gastric cytoprotective effect amongst other health benefits. 2, 3 The concentration of individual terpenes varies by strain, can be anywhere from 0.1 to 1.5% of its total dry weight, and can vary depending on harvest time, drying and storage conditions. 1, 4 Terpene levels can decrease over time, and after three months of storage, can reduce terpene levels by more than half.4 The decrease in terpene amount over time varies for different terpenes.
Susan C, Trapp
http://susanctrapp.com/
Terpene expert
Not Cannabis specific
Stability of Essential Oils: A Review.
Turek, C., & Stintzing, F. C.
Comprehensive Reviews in Food Science and Food Safety, 12(1), 40–53.(2013).
doi:10.1111/1541-4337.12006
In recent years, consumers have developed an ever-increasing interest in natural products as alternatives for artificial additives or pharmacologically relevant agents. Among them, essential oils have gained great popularity in the food, cosmetic, as well as the pharmaceutical industries. Constituting an array of many lipophilic and highly volatile components derived from a great range of different chemical classes, essential oils are known to be susceptible to conversion and degradation reactions. Oxidative and polymerization processes may result in a loss of quality and pharmacological properties. Despite their relevance for consumers, there is a paucity of information available addressing this issue. Therefore, the present review provides a comprehensive summary on possible changes in essential oils and factors affecting their stability. Focusing on individual essential oils, the various paths of degradation upon exposure to extrinsic parameters are outlined. Especially temperature, light, and oxygen availability are recognized to have a crucial impact on essential oil integrity. Finally, analytical methods to assess both genuine as well as altered essential oil profiles are evaluated with respect to their suitability to track chemical alterations. It is believed that only a careful inspection of essential oils by a set of convenient methods allows profound quality assessment that is relevant to producers and consumers alike.
Not Cannabis specific
Structure of limonene synthase, a simple model for terpenoid cyclase catalysis
David C. Hyatt, Buhyun Youn, Yuxin Zhao, Bindu Santhamma, Robert M. Coates, Rodney B. Croteau and ChulHee Kang
PNAS March 27, 2007 _ vol. 104 _ no. 13
DOI: 10.1073_pnas.0700915104
https://www.pnas.org/content/pnas/104/13/5360.full.pdf
The crystal structure of (4S)-limonene synthase from Mentha spicata, a metal ion-dependent monoterpene cyclase that catalyzes the coupled isomerization and cyclization of geranyl diphosphate, is reported at 2.7-Å resolution in two forms liganded to the substrate and intermediate analogs, 2-fluorogeranyl diphosphate and 2- fluorolinalyl diphosphate, respectively. The implications of these findings are described for domain interactions in the homodimer and for changes in diphosphate–metal ion coordination and substrate binding conformation in the course of the multistep reaction.
Systems and methodologies for rapid and robust Cannabis Terpene Analysis 5 minute analysis time = more productivity
Ron Honnold, Agilent
Conference: 2017 Cannabis Science Conference At: Portland, OR August 2017
DOI: 10.13140/RG.2.2.34481.89441
Headspace-Gas Chromatography Flame Ionization Detector with Mass Spectrometer methodology enhancements allow full scan acquisition with a 5 minute run time with separation using both FID and MS for quantitation.
Taming THC: potential cannabis synergy and phytocannabinoid terpenoid entourage effects
Ethan B Russo
British Journal of Pharmacology (2011) 163 1344–1364
doi: 10.1111/j.1476-5381.2011.01238.x
Tetrahydrocannabinol (THC) has been the primary focus of cannabis research since 1964, when Raphael Mechoulam isolated and synthesized it. More recently, the synergistic contributions of cannabidiol to cannabis pharmacology and analgesia have been scientifically demonstrated. Other phytocannabinoids, including tetrahydrocannabivarin, cannabigerol and cannabichromene, exert additional effects of therapeutic interest. Innovative conventional plant breeding has yielded cannabis chemotypes expressing high titres of each component for future study. This review will explore another echelon of phytotherapeutic agents, the cannabis terpenoids: limonene, myrcene, ??pinene, linalool, ??caryophyllene, caryophyllene oxide, nerolidol and phytol. Terpenoids share a precursor with phytocannabinoids, and are all flavour and fragrance components common to human diets that have been designated Generally Recognized as Safe by the US Food and Drug Administration and other regulatory agencies. Terpenoids are quite potent, and affect animal and even human behaviour when inhaled from ambient air at serum levels in the single digits ng·mL?1. They display unique therapeutic effects that may contribute meaningfully to the entourage effects of cannabis?based medicinal extracts. Particular focus will be placed on phytocannabinoid?terpenoid interactions that could produce synergy with respect to treatment of pain, inflammation, depression, anxiety, addiction, epilepsy, cancer, fungal and bacterial infections (including methicillin?resistant Staphylococcus aureus). Scientific evidence is presented for non?cannabinoid plant components as putative antidotes to intoxicating effects of THC that could increase its therapeutic index. Methods for investigating entourage effects in future experiments will be proposed. Phytocannabinoid?terpenoid synergy, if proven, increases the likelihood that an extensive pipeline of new therapeutic products is possible from this venerable plant.
Tandem mass spectrometric quantification of 93 terpenoids in Cannabis using static headspace (SHS) injections.
Shapira, A., Berman, P., Futoran, K., Guberman, O., & Meiri, D.
Analytical Chemistry. (2019).
doi:10.1021/acs.analchem.9b02844
The therapeutic effect of Cannabis largely depends on the content of its pharmacologically active secondary metabolites, mainly phytocannabinoids, flavonoids, and terpenoids. Recent studies suggest there are therapeutic effects of specific terpenoids as well as synergistic effects with other active compounds in the plant. Although Cannabis contains an overwhelming milieu of terpenoids, only a limited number are currently reported and used for metabolic analysis of Cannabis chemovars. In this study, we report the development and validation of a method for simultaneous quantification of 93 terpenoids in Cannabis air-dried inflorescences and extracts. This method employs the full evaporation technique via a static headspace sampler, followed by gas chromatography?mass spectrometry (SHS-GC/MS/MS). In the validation process, spiked terpenoids were quantified with acceptable repeatability, reproducibility, sensitivity, and accuracy. Three medical Cannabis chemovars were used to study the effect of sample preparation and extraction methods on terpenoid profiles. This method was further applied for studying the terpenoid profiles of 16 different chemovars acquired at different dates. Our results demonstrate that sample preparation methods may significantly impact the chemical fingerprint compared to the nontreated Cannabis. This emphasizes the importance of performing SHS extraction in order to study the natural terpenoid contents of chemovars. We also concluded that most inflorescences expressed relatively unique terpenoid profiles for the most pronounced terpenoids, even when sampled at different dates, although absolute concentrations may vary due to aging. The suggested method offers an ideal tool for terpenoid profiling of Cannabis and sets the scene for more comprehensive works in the future.
Not Cannabis specific
Taxadiene synthase structure and evolution of modular architecture in terpene biosynthesis.
Köksal, M., Jin, Y., Coates, R. M., Croteau, R., & Christianson, D. W.
Nature, 469(7328), 116–120.(2010).
doi:10.1038/nature09628
With more than 55,000 members identified so far in all forms of life, the family of terpene or terpenoid natural products represents the epitome of molecular biodiversity. A well-known and important member of this familyis the polycyclic diterpenoid Taxol (paclitaxel), which promotes tubulin polymerization1 and shows remarkable efficacy in cancer chemotherapy2 . The first committed step of Taxol biosynthesis in the Pacific yew (Taxus brevifolia) 3 is the cyclization of the linear isoprenoid substrate geranylgeranyl diphosphate (GGPP) to form taxa-4(5),11(12)diene4 , which is catalysed by taxadiene synthase5 . The full-length form of this diterpene cyclase contains 862 residues, but a roughly 80-residue amino-terminal transit sequence is cleaved on maturation in plastids6 . We now report the X-ray crystal structure of a truncation variant lacking the transit sequence and an additional 27 residues at the N terminus, hereafter designated TXS. Specifically, we have determined structures of TXS complexed with 13-aza-13,14-dihydrocopalyl diphosphate (1.82 A? resolution) and 2-fluorogeranylgeranyl diphosphate (2.25 A? resolution). The TXS structure reveals a modular assembly of three ahelical domains. The carboxy-terminal catalytic domain is a class I terpenoid cyclase, which binds and activates substrate GGPP with a three-metal ion cluster. The N-terminal domain and a third ‘insertion’ domain together adopt the fold of a vestigial class II terpenoid cyclase. A class II cyclase activates the isoprenoid substrate by protonation instead of ionization, and the TXS structure reveals a definitive connection between the two distinct cyclase classes in the evolution of terpenoid biosynthesis
TERPEDIA The Terpene Encyclopedia
https://terpedia.com/
Not Specific to Cannabis
Terpene biosynthesis in glandular trichomes of hop.
Wang G, Tian L, Aziz N, Broun P, Dai X, He J, King A, Zhao PX, Dixon RA.
Plant Physiol. 2008 Nov;148(3):1254-66.
doi: 10.1104/pp.108.125187.
Hop (Humulus lupulus L. Cannabaceae) is an economically important crop for the brewing industry, where it is used to impart flavor and aroma to beer, and has also drawn attention in recent years due to its potential pharmaceutical applications. Essential oils (mono- and sesquiterpenes), bitter acids (prenylated polyketides), and prenylflavonoids are the primary phytochemical components that account for these traits, and all accumulate at high concentrations in glandular trichomes of hop cones. To understand the molecular basis for terpene accumulation in hop trichomes, a trichome cDNA library was constructed and 9,816 cleansed expressed sequence tag (EST) sequences were obtained from random sequencing of 16,152 cDNA clones. The ESTs were assembled into 3,619 unigenes (1,101 contigs and 2,518 singletons). Putative functions were assigned to the unigenes based on their homology to annotated sequences in the GenBank database. Two mono- and two sesquiterpene synthases identified from the EST collection were expressed in Escherichia coli. Hop MONOTERPENE SYNTHASE2 formed the linear monterpene myrcene from geranyl pyrophosphate, whereas hop SESQUITERPENE SYNTHASE1 (HlSTS1) formed both caryophyllene and humulene from farnesyl pyrophosphate. Together, these enzymes account for the production of the major terpene constituents of the hop trichomes. HlSTS2 formed the minor sesquiterpene constituent germacrene A, which was converted to beta-elemene on chromatography at elevated temperature. We discuss potential functions for other genes expressed at high levels in developing hop trichomes.
Terpene Comparison chart
Yewande Okuleye
DOI: 10.13140/RG.2.2.36575.87203
Conference: Psychoactive Supper At: London May 2016
Affiliation: University of Leicester
This visual/odour profile card was designed to communicate ideas about the ubiquity of terpenes in plants. The comparison between food substances and cannabis asked question about the cultural meanings which cannabis has engendered due to the international drug regime
Terpene Contents Differ in Flowers and Supercritical Co2 Extract
Cara Wietstock
https://terpenesandtesting.com/terpe...l-co2-extract/
From how to best extract them to their best use medicinally; terpenes have been the topic of conversation for everyone from the consumer to the research analyst. This fall, Dr. Michelle Sexton conducted a comparative study [1] of the cannabinoid and terpene content in both cannabinoid flowers and supercritical CO2 extract. Their findings support the idea that the current nomenclature and consumer labeling habits do not help the consumer find the product that they’re looking for.The quantification of seven cannabinoids analysts in the project used a validatedhigh-performance liquid chromatography/diode array detector methodology. To identify which of 42 terpenes were in each sample they utilized internal gas chromatography-mass spectrometry method. What they found was shocking. Between the flower and the extract, the terpenoid and cannabinoid contents were significantly different.Cannabinoid potency in extracts increased by factors of 4.0 for cannabidiol and 3.2 for ?-9 tetrahydrocannabinol when compared to the flower used for start materials. The study also found that monoterpenes were lost in the extraction process. Ketone, monoterpene alcohols, sesquiterpenes all showed an increase in the supercritical CO2 extract as compared to the results from the original dried, cured cannabis flowers.
Terpene Exhaust Emissions and Impact Ozone Modeling from Cannabis Plants at Commercial Indoor Cultivation Facilities in Colorado
Kaitlin Urso,Alicia Frazier, Sara Heald & Andrey Khlystov
DOI: 10.1080/10962247.2022.2046206
https://www.tandfonline.com/doi/full...eedAccess=true
In 2019, an air emissions field sampling study was conducted by the Colorado Department of Public Health and Environment (CDPHE) Air Pollution Control Division (APCD) at three commercial cannabis cultivation facilities. The goal of the study was to quantify biogenic-terpene volatile organic compound (VOC) emissions from growing cannabis at cultivation facility exhaust points to estimate a VOC emission rate by a top-down approach. The resulting VOC emission rates were then used in combination with 2019 commercial cannabis cultivation facility biomass production volumes (harvest weight) and cultivation locations from the Colorado Department of Revenue’s Marijuana Enforcement Division (MED) to model the potential ozone and PM2.5 formation impacts of the cannabis industry in the Denver Metro North Front Range (DM/NFR) Ozone Nonattainment Area (NAA).
Despite cannabis cultivation facilities’ high nuisance odors, this study found the biogenic VOC emissions rate from the sampled indoor facilities to be low (2.13 lbs to 11.12 lbs of VOC/ton of cannabis harvested), even at large production facilities. The dominant terpenes from this sampling study present in most samples were β-caryophyllene, D-limonene, terpinolene, α-pinene, β-pinene and β-myrcene respectively by concentration. Interestingly, the cannabis emissions exhaust profile lacked isoprene, a terpene commonly emitted from other plants that is highly reactive and has great potential to contribute to ozone formation [Sharkey et al 2007, Wang et al. 2019]. The low biogenic VOC emissions rate and the lack of isoprene from the cannabis cultivation facilities sampled resulted in a very low to negligible impact on both ozone formation (0.005% - 0.009% increase in ozone from cannabis cultivation) and PM2.5 formation (largest maximum 24-hour PM2.5 difference of 0.009 µg/m3) in the DM/NFR NAA.
Find PDF
Terpene Extraction by Ultrasonics
Derek Johnson
Extraction Magazine
https://extractionmagazine.com/2021/...y-ultrasonics/
There are many techniques for extracting terpenes, each with its own advantages. Of them all, ultrasonic extraction (ultrasonics) offers many benefits, which is why it’s quickly becoming a preferred extraction method for botanicals including cannabis and hops.
The very nature of ultrasonic extraction makes it an efficient and eco-friendly method of production. A solvent, water, or oil is used as a medium to hold the material meant to undergo extraction. Ultrasound extraction works with any solvent, including pressurized solvents like supercritical carbon dioxide and hydrocarbons like butane.
Ultrasonic extraction uses sound energy to create waves and vibrations greater than 20 kilohertz. These waves and vibrations are capable of agitating materials intended for extraction and can lead to superior terpene and cannabinoid extract yields. The method helps catalyze an extraction by deagglomerating biomass particles and reducing their size.
During the extraction, a process called cavitation takes place, which leads to the production of innumerable tiny bubbles that expand and implode rapidly and with great force. The energy generated is what destroys the cell walls of the material undergoing extraction, causing the desired compounds to flow more freely from the cells into the liquid or gel holding the source material.
Terpene Extractors Milestone
https://www.milestonesrl.com/product...ave-extraction
Get links for all
Cannabis-Science-and-Technology
Cannabis-Terpene-CAT348EN
ETHOS X Microwave Green Extraction of Natural Products – Milestone
Improving The Quality And Efficiency Of Terpene Extraction From Cannabis Plant
Isolation Of Strain Specific HIGH QUALITY TERPENES Through Microwave Assisted Extraction
MAC-75
Maximize Your Cannabis Terpene Profile
Microwave Extraction Systems - Milestone Inc. - for GC, GC-MS & HPLC sample prep
Milestones Technology Provides Cannabis Processor Quality And Efficiency In Terpene Extraction
Find Pdf's
Terpene Isolation Could Be The Future Of Cannabis
Drake Dorm
https://www.medicaljane.com/2013/10/...e-of-cannabis/
Terpenes are aromatic compounds that are largely responsible for the smell and taste of cannabis. Terpenes are believed to interact synergistically with cannabinoids to improve the efficacy of medical marijuana.
With that said, relatively little is known about terpenes as far as cannabis is concerned. Most often, the cannabis community thinks of terpenes solely as a source of aroma and flavor in their favorite strain of cannabis. However, there are a number of possible applications for terpenes that are unknown to many people who consider themselves a cannabis connoisseur.
For instance, many third-party testing facilities lab-test various cannabis strains and infused products. They are then able to use their database to determine what the average terpene profile for each strain is, and compare future test samples with these standards. The same process could potentially help determine the genetic lineage of a particular strain in a case where it is unknown.
Even further, terpenes are of interest to a number of investigators in their own right. A great deal of focus in the cannabis industry has turned towards the concept of isolating terpenes and the potential application of such a process. In fact, there are rumors about a number of pure terpene products expected to be on the market in the very near future – most notably from The Werc Shop and Cannabis Biotech.
Terpene Synthases and Terpene Variation in Cannabis sativa.
Booth, J. K., Yuen, M. M. S., Jancsik, S., Madilao, L., Page, J., & Bohlmann, J.
Plant Physiology, pp.00593.2020. (2020).
doi:10.1104/pp.20.00593
Cannabis (Cannabis sativa) resin is the foundation of a multi-billion dollar medicinal and recreational plant bioproducts industry. Major components of the cannabis resin are the cannabinoids and terpenes. Variations of cannabis terpene profiles contribute much to the different flavor and fragrance phenotypes that affect consumer preferences. A major problem in the cannabis industry is the lack of proper metabolic characterization of many of the existing cultivars, combined with sometimes incorrect cultivar labeling. We characterized foliar terpene profiles of plants grown from 32 seed sources and found large variation both within and between sets of plants labeled as the same cultivar. We selected five plants representing different cultivars with contrasting terpene profiles for clonal propagation, floral metabolite profiling and trichome-specific transcriptome sequencing. Sequence analysis of these five cultivars and the reference genome of the Purple Kush (PK) cultivar revealed a total of 33 different cannabis terpene synthase (CsTPS) genes as well as variations of the CsTPS gene family and differential expression of terpenoid and cannabinoid pathway genes between cultivars. Our annotation of the PK reference genome identified 19 complete CsTPS gene models, and tandem arrays of isoprenoid and cannabinoid biosynthetic genes. An updated phylogeny of the CsTPS gene family showed three cannabis-specific clades, including a clade of sesquiterpene synthases within the TPS-b subfamily that typically contains mostly monoterpene synthases. The CsTPSs described and functionally characterized here include 13 that had not been previously characterized and collectively explain a diverse range of cannabis terpenes.
Terpene synthases from Cannabis sativa
Judith K. Booth, Jonathan E. Page, Jorg Bohlmann
PLoS ONE 12(3): e0173911 2017
Doi: 10.1371/journal.pone.0173911
Cannabis (Cannabis sativa) plants produce and accumulate a terpene-rich resin in glandular trichomes, which are abundant on the surface of the female inflorescence. Bouquets of different
monoterpenes and sesquiterpenes are important components of cannabis resin as they define some of the unique organoleptic properties and may also influence medicinal qualities of different cannabis strains and varieties. Transcriptome analysis of trichomes of the cannabis hemp variety ‘Finola’ revealed sequences of all stages of terpene biosynthesis. Nine cannabis terpene synthases (CsTPS) were identified in subfamilies TPS-a and TPS-b. Functional characterization identified mono- and sesqui-TPS, whose products collectively comprise most of the terpenes of ‘Finola’ resin, including major compounds such as ?-myrcene, (E)-?-ocimene, (-)-limonene, (+)-?-pinene, ?-caryophyllene, and ?-humulene. Transcripts associated with terpene biosynthesis are highly expressed in trichomes compared to non-resin producing tissues. Knowledge of the CsTPS gene family may offer opportunities for selection and improvement of terpene profiles of interest in different cannabis strains and varieties.
NOT CANNABIS SPECIFIC
TERPENES
Eberhard Breitmaier
https://epdf.pub/terpenes1f1560e0e08...7a851619 2.html
Terpenes and derivatives as a new perspective for pain treatment: a patent review
Adriana G Guimaraes, Mairim R Serafini & Lucindo J Quintans-Junior
Expert Opin. Ther. Patents (2013) 24(3)
DOI: 10.1517/13543776.2014.870154
Introduction: Terpenes are natural compounds found in several organisms belonging to the animal and plant kingdoms. They constitute the largest class of natural products with > 55,000 known compounds structurally diversified. Several studies have attributed to this big family of compounds a range of pharmacological properties, such as anticancer, antimicrobial, antifungal, antiviral, antihyperglycemic, analgesic, anti-inflammatory and antiparasitic.
Areas covered: In this review, the authors summarize therapeutic patent applications concerning the employment of terpenes for pain relief, focusing on the perspective for these compounds to become candidates for new drugs intended to control painful syndromes.
Expert opinion: Over years of tremendous academic and industrial investment in the characterization of the analgesic action of terpenes, there was the development of a successful product that has been well-accepted clinically. Furthermore, there is still hope that new therapeutic options for the control of painful syndromes will be developed from terpenes, which have been shown to be great candidates for this purpose because of the range of pharmacological mechanisms in important target sites
NOT CANNABIS SPECIFIC
Terpenes and Terpenoids
Shagufta Perveen, Areej Al-Taweel
https://www.researchgate.net/publica...and_Terpenoids
Natural products are the compounds which isolate from different natural sources such as plants, animals, microbes, insects, plant pathogens, and endophytes and marine. These are known as secondary metabolites since they are formed due to the enzymatic resections of primary metabolites (amino acids, sugars, vitamins, etc.). Terpenes belong to the biggest class of secondary metabolites and basically consist of five carbon isoprene units which are assembled to each other (many isoprene units) by thousands of ways. Terpenes are simple hydrocarbons, while terpenoids are modified class of terpenes with different functional groups and oxidized methyl group moved or removed at various positions. Terpenoids are divided into monoterpenes, sesquiterpenes, diterpenes, sesterpenes, and triterpenes depending on its carbon units (Figure 1). Most of the terpenoids with the variation in their structures are biologically active and are used worldwide for the treatment of many diseases. Many terpenoids inhibited different human cancer cells and are used as anticancer drugs such as Taxol and its derivatives. Many flavorings and nice fragrances are consisting on terpenes because of its nice aroma. Terpenes and its derivatives are used as antimalarial drugs such as artemisinin and related compounds. Meanwhile, terpenoids play a diverse role in the field of foods, drugs, cosmetics, hormones, vitamins, and so on. This chapter provides introduction and information on the bioactive terpenes isolated currently from different natural sources
Terpenoids From Cannabis Do Not Mediate an Entourage Effect by Acting at Cannabinoid Receptors.
Finlay, D. B., Sircombe, K. J., Nimick, M., Jones, C., & Glass, M.
Frontiers in Pharmacology, 11.(2020).
doi:10.3389/fphar.2020.00359
The entourage effect was a proposed explanation for biological observations that endocannabinoid ligand activities can be modified by other lipids released from cells at the same time. An increasing volume of anecdotal reports and interest in the plant have provoked research into the activity of minor chemical constituents of the plant—including volatile terpenoids such as myrcene, a- and b- pinene, b-caryophyllene, and limonene. However, to date, no clear interaction has been identified. The current study was designed to determine whether terpenes in the cannabis plant have detectable receptor-mediated activity, or modify the activity of D9 -tetrahydrocannabinol, cannabidiol, or the endocannabinoid 2-arachidonylglycerol at the cannabinoid receptors. In addition, we have utilized a standard radioligand binding paradigm with ability to detect orthosteric and allosteric interactions of test compounds. With the possible exception of a weak interaction of b-caryophyllene with CB2, no data were produced to support the hypothesis that any of the five terpenes tested (either alone or in mixtures) have direct interactions with CB1 or CB2, as the binding of radioligand ([3 H]-CP55,940), D9 - tetrahydrocannabinol, and cannabidiol were unaltered by the presence of terpenes. Similarly, terpene functional effects were also not detected, either alone or in combination with D9 -tetrahydrocannabinol, cannabidiol, or 2-arachidonoylglycerol. This study adds to the evidence that the putative entourage effect cannot be explained by direct effects at CB1 or CB2.
Terpenes in Cannabis sativa – from plant genome to humans.
Booth JK, Bohlmann J (2019)
Plant Sci 284: 67-72
Doi: 10.1016/j.plantsci.2019.03.022
Cannabis sativa (cannabis) produces a resin that is valued for its psychoactive and medicinal properties. Despite being the foundation of a multi-billion dollar global industry, scientific knowledge and research on cannabis is lagging behind compared to other high-value crops. This is largely due to legal restrictions that have prevented many researchers from studying cannabis, its products, and their effects in humans. Cannabis resin contains hundreds of different terpene and cannabinoid metabolites. Many of these metabolites have not been conclusively identified. Our understanding of the genomic and biosynthetic systems of these metabolites in cannabis, and the factors that affect their variability, is rudimentary. As a consequence, there is concern about lack of consistency with regard to the terpene and cannabinoid composition of different cannabis ‘strains’. Likewise, claims of some of the medicinal properties attributed to cannabis metabolites would benefit from thorough scientific validation.
Terpenes in Cannabis: Solving the Puzzle of How to Predict Taste and Smell.
Roell, M.-S.
Plant Physiology, 184(1), 8–9. (2020).
doi:10.1104/pp.20.00919
In this issue of Plant Physiology, Booth et al. (2020) provide the framework for future breeding efforts to produce cannabis fragrance and flavor features demanded by consumers. Specifically, they analyzed terpene profiles of eight cannabis cultivars and characterized 13 new cannabis terpene synthases. In plants, terpenes form a diverse group of hydrocarbon-based metabolites estimated to encompass thousands of different molecules (Pichersky and Raguso, 2018). Terpenes have diverse roles. They function as primary cellular components, e.g. as hormones or antioxidants, and they are indispensable for ecological interactions, e.g. signaling and defense against herbivores (Pichersky and Raguso, 2018). In cannabis, more than 100 different terpenes have been identified that define odor and flavor of different cultivars (Rothschild et al., 2005; Andre et al., 2016). Terpene- and cannabinoid-biosynthesis depend on the five carbon building block isopentenyl pyrophosphate (IPP; Fig. 1). IPP is produced by the plastidial methylerythritol phosphate pathway and the cytosolic mevalonate pathway. Metabolic fluxes within both pathways contribute to the substrate pools available for terpene synthases (TPSs). TPSs produce the diversity of cyclic and acyclic terpene core structures, using geranyl diphosphate or farnesyl diphosphate for monoterpene or sesquiterpene synthesis, respectively (Fig. 1). The accurate predicting and design of cannabis terpene profiles requires understanding of TPS, the key enzyme in terpene biosynthesis (Fig. 1). Booth et al. (2020) identified 19 TPS gene models in the ‘Purple Kush’ cannabis reference genome. TPS genes show multicopy gene clustering, a common phenomenon previously observed for genes of the IPP and cannabinoid biosynthetic pathways (Taura et al., 2009). Foliar terpene profiling of eight cannabis cultivars revealed a total of 48 different terpenes with three monoterpenes (myrcene, a?pinene, and limonene) and two sesquiterpenes (b?caryophyllene and a?humulene) present in all cultivars. In six selected cultivars, monoterpenes accumulated during the life cycle, in tissues including leaves, juvenile flowers, and adult flowers. Using trichome transcriptome profiling, Booth et al. (2020) identified 33 TPS genes among the selected six cultivars. Further, 13 new TPSs were biochemically characterized regarding product formation using both geranyl diphosphate and farnesyl diphosphate as substrates. Overall TPS specificity varied between the production of a single mono- or sesquiterpene to as many as 13 different sesquiterpenes produced by a single TPS enzyme. With these results, simple assumptions regarding TPS transcript abundance and terpene profiles can be made. However, spatiotemporal profiling of TPS transcript levels and terpene quantities will be necessary for more accurate predictions. Integrating the 13 TPSs characterized in this study with previously characterized TPS brings the total number to 30 known TPSs across 14 different cannabis cultivars (Booth et al., 2017; Zager et al., 2019; Livingston et al., 2020). Harmonizing trichome transcriptomics tools, knowledge of TPS function, and terpene profiling sets the framework for cannabis breeders to predictively shape and design terpene composition on demand
LIST: Terpenes in vape
EPA
https://comptox.epa.gov/dashboard/ch...s/VAPETERPENES
Description: Terpenes are organic compounds found in the marijuana plant that give strains their distinct aromatic and flavor profiles. They are now being isolated and concentrated into oils for individual vaping.
Number of Chemicals: 37
Terpenes May Improve Effectiveness of Medical Marijuana
Drake Dorm
https://www.medicaljane.com/2013/09/...cal-marijuana/
Terpenes Influence the Synergy Effect of Cannabis
As we know, science has identified and characterized the molecular structure of around 20,000 terpenes, which makes it the largest category of plant chemicals. These aromatic compounds are found in the essential oils of plants and flowers, and plenty of studies have been done on their effects. Of the 20,000 identified terpenes, about 140 of these have been found in cannabis. Only a few of them appear in high concentrations, but they have been found to have a number of benefits. Terpenes play very important individual roles, but recent research also suggests an “entourage effect” may exist. In his 2011 study, Ethan Russo (GW Pharmaceuticals), discussed the interaction between terpenes and cannabinoids. In fact, Russo points out that terpenes likely played a role in a number of ancient antidotes for the less desirable effects of THC. For instance, citrus fruits (high in limonene) were used as a “cannabis antidote” in 10th century Persia. Other ancient antidotes include calamus plant roots and pine nuts (high in pinene), as well as black pepper (high in caryophyllene and myrcene).
Not directly Cannabis related
Terpenes solubility in water and their environmental distribution.
Martins, M. A. R., Silva, L. P., Ferreira, O., Schröder, B., Coutinho, J. A. P., & Pinho, S. P.
Journal of Molecular Liquids, 241, 996–1002.(2017).
doi:10.1016/j.molliq.2017.06.099
Terpenes and terpenoids belong to the largest and most diverse class of natural products. Due to the increasing importance of their applications and the emerging perception of their impact on the environment, the available physico-chemical characterization is insufficient. In this work the water solubility of geraniol, linalool, DL-citronellol, thymol, eugenol, carvacrol and pcymene, in the temperature range from (298.15 to 323.15) K, and at atmospheric pressure, is studied. Due to the low solubility of these compounds a novel technique was adopted for their measurements and validated using the aqueous solubility data for sparingly soluble aromatic compounds. The thermodynamic properties of solution were derived from the experimental data at infinite dilution. It is shown that the solubility of terpenes in water is an endothermic process confirming the existence of UCST phase diagrams, and only for carvacrol and eugenol is entropically driven. The experimental information is shown in a two-dimensional chemical space diagram providing indications to their probable distribution in the environment once released.
Terpenes/Terpenoids in Cannabis: Are They Important?
Lumír Ond?ej, Hanuš, Yotam Hod
Med Cannabis Cannabinoids, 1-36 (2020)
DOI: 10.1159/000509733
Cannabis sativa plant has not only cannabinoids as crucial compounds but also the other compounds that play important role as synergistic and/or entourage compound. Cannabis/hemp plant materials and essential oils were analyzed with the help of gas chromatography/mass spectrometry detector for the content of terpenes and terpenoids. The main terpenes/terpenoids and their abundance in the samples were evaluated. Results of this study will be helpful in the next evaluation of these compound in mixture with cannabinoids and their importance in medical treatment.
Terpenoid biosynthesis in trichomes—current status and future opportunities
B. Markus Lange and Glenn W. Turner
Plant Biotechnology Journal (2012), pp. 1–21
doi: 10.1111/j.1467-7652.2012.00737.x
Glandular trichomes are anatomical structures specialized for the synthesis of secreted natural products. In this review we focus on the description of glands that accumulate terpenoid essential oils and oleoresins. We also provide an in-depth account of the current knowledge about the biosynthesis of terpenoids and secretion mechanisms in the highly specialized secretory cells of glandular trichomes, and highlight the implications for metabolic engineering
efforts.
Terpenoid chemoprofiles distinguish drug-type Cannabis sativa L. cultivars in Nevada.
Orser C, Johnson S, Speck M, Hilyard A, Afia I (2018)
Nat Prod Chem Res 6: 304
doi:10.4172/2475-7675.1000304
https://www.researchgate.net/publica...vars_in_Nevada
An unintended consequence of state-mandated cannabis testing regulations has been the resulting database from the analysis of thousands of individual cannabis flower samples from artificially restricted geographical regions. The resulting detailed chemical database can serve as the basis for the development of a chemotaxonomic classification scheme outside of conjectural cultivar naming by strain. Chemotaxonomic classification schemes for cannabis cultivars have previously been reported by others based largely on cannabis strains grown in California under an unregulated testing environment or in Europe from strains grown by a single cultivator. In this study 2,237 individual cannabis flower samples, representing 204 individual strains across 27 cultivators in a tightly regulated Nevada cannabis testing market, were analyzed across 11 cannabinoids and 19 terpenoids. Even though 98.3% of the samples were from Type I cannabis strains by cannabidiolic acid/tetrahydrocannabinolic acid (THCA) ratio of <0.5 CBDA, principal component analysis (PCA) of the combined dataset resulted in three distinct clusters that were distinguishable by terpene profiles alone. Further dissection of individual strains by cultivators within clusters revealed striking fidelity of terpenoid profiles and also revealed a few outliers. We propose that three terpenoid cluster assignments account for the diversity of drug type cannabis strains currently being grown in Nevada.
Terpenoid Chemoprofiles Distinguish Drug-type Cannabis sativa L. Cultivars in Nevada
Philippe Henry, Cindy Orser, Steve Johnson, Aaron Hilyard, S Thoslon, A Everett, Mark D Speck
Conference: The Emerald Conference February 2018 (Poster)
DOI: 10.13140/RG.2.2.20022.40006
https://www.researchgate.net/publica...vars_in_Nevada
One consequence of state-mandated cannabis testing regulations has been the resulting database from the analysis of thousands of individual cannabis flower samples from artificially restricted geographical regions. The resulting detailed chemical database can serve as the basis for the development of a chemotaxonomic classification scheme outside of conjectural cultivar naming by strain. Chemotaxonomic classification schemes for cannabis cultivars have previously been reported by others based largely on cannabis strains grown in unregulated testing environments or in Europe from strains grown by a single cultivator. In this study 2,237 individual cannabis flower samples, representing 204 individual strains across 27 cultivators in a tightly regulated Nevada cannabis testing market, were analyzed across 11 cannabinoids and 19 terpenoids. Even though 98.3% of the samples were from Type I cannabis strains based on CBDA/THCA cannabinoid ratio, principal component analysis (PCA) of the combined dataset resulted in three distinct clusters that were distinguishable by terpene profiles alone. Further dissection of individual strains by cultivators within clusters revealed striking fidelity of terpenoid profiles but also revealed a few outliers. We propose that three terpenoid cluster assignments, and as few as three terpenoids, account for the diversity of drug-type cannabis strains currently being grown in Nevada.
Not Cannabis specific
Terpenoid synthase structures: a so far incomplete view of complex catalysis.
Gao, Y., Honzatko, R. B., & Peters, R. J.
Natural Product Reports, 29(10), 1153. (2012).
doi:10.1039/c2np20059g
The complexity of terpenoid natural products has drawn significant interest, particularly since their common (poly)isoprenyl origins were discovered. Notably, much of this complexity is derived from the highly variable cyclized and/or rearranged nature of the observed hydrocarbon skeletal structures. Indeed, at least in some cases it is difficult to immediately recognize their derivation from poly-isoprenyl precursors. Nevertheless, these diverse structures are formed by sequential elongation to acyclic precursors, most often with subsequent cyclization and/or rearrangement. Strikingly, the reactions used to assemble and diversify terpenoid backbones share a common carbocationic driven mechanism, although the means by which the initial carbocation is generated does vary. High-resolution crystal structures have been obtained for at least representative examples from each of the various types of enzymes involved in producing terpenoid hydrocarbon backbones. However, while this has certainly led to some insights into the enzymatic structure– function relationships underlying the elongation and simpler cyclization reactions, our understanding of the more complex cyclization and/or rearrangement reactions remains limited. Accordingly, selected examples are discussed here to demonstrate our current understanding, its limits, and potential ways forward.
Terpenoids and Phytocannabinoids Co-Produced in Cannabis Sativa Strains Show Specific Interaction for Cell Cytotoxic Activity.
Namdar, D., Voet, H., Ajjampura, V., Nadarajan, S., Mayzlish-Gati, E., Mazuz, M., … Koltai, H.
Molecules, 24(17), 3031. (2019).
doi:10.3390/molecules24173031
: Mixtures of different Cannabis sativa phytocannabinoids are more active biologically than single phytocannabinoids. However, cannabis terpenoids as potential instigators of phytocannabinoid activity have not yet been explored in detail. Terpenoid groups were statistically co-related to certain cannabis strains rich in ? 9 -tetrahydrocannabinolic acid (THCA) or cannabidiolic acid (CBDA), and their ability to enhance the activity of decarboxylase phytocannabinoids (i.e., THC or CBD) was determined. Analytical HPLC and GC/MS were used to identify and quantify the secondary metabolites in 17 strains of C. sativa, and correlations between cannabinoids and terpenoids in each strain were determined. Column separation was used to separate and collect the compounds, and cell viability assay was used to assess biological activity. We found that in “high THC” or “high CBD” strains, phytocannabinoids are produced alongside certain sets of terpenoids. Only co-related terpenoids enhanced the cytotoxic activity of phytocannabinoids on MDA-MB-231 and HCT-116 cell lines. This was found to be most effective in natural ratios found in extracts of cannabis inflorescence. The correlation in a particular strain between THCA or CBDA and a certain set of terpenoids, and the partial specificity in interaction may have influenced the cultivation of cannabis and may have implications for therapeutic treatments
Terpenoids Biotransformation in Mammals III: Biotransformation of a-Pinene, ,8-Pinene, Pinane, 3-Carene, Carane, Myrcene, and p-Cymene in Rabbits
T. ISHIDA, Y. ASAKAWA, T. TAKEMOTO, and T. ARATANT
Journal of Pharmeceutlcal Sciences Vol. 70, No. 4, April 1981
DOI: 10.1002/JPS.2600700417
The biotransformation of (+)-, (-)-, and (*)-a-pinenes, (-)-/?-pinene (nopinene), (-)-cis-pinane, (+)-3-carene, (-)-cis-carane, myrcene, and p-cymene in rabbits was investigated. The major metabolites were as follows: (-)-trans-verbenol from (+)-, (-)-, and (&Ia-pinenes; (-)-10-pinanol and (-)-l-p-menthene-7,8-diol from (-)- /?-pinene; (-)-a-terpineol and (-)-trans-sobrerol from (-)Aspinane; (-)-m-rnentha-4,6-dien-8-01, 3-caren-9-01, (-)-3-carene-9-carboxylic acid, and 3-carene-9,10-dicarboxylic acid from (+)-3-carene; carane9,lO-dicarboxylic acid from (-)-cis-carane; and myrcene-3(10)-glycol, myrcene-1,2-glycol, uroterpenol, and p-cymene-9-carboxylic acid from p-cymene. These metabolisms include allylic oxidation, epoxidation, stereoselective gem-dimethyl hydroxylation and its oxidation, cleavage of a conjugated double bond by epoxidation, and regioselective oxidation, some of which are not found usually in chemical reactions, and due to which various new compounds were determined. This biotransformation of the monoterpene hydrocarbons gave some insect pheromones in high yield.
Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Actions of Phytocannabinoids or Endocannabinoids on TRPA1 and TRPV1 Channels.
Heblinski, M., Santiago, M., Fletcher, C., Stuart, J., Connor, M., McGregor, I. S., & Arnold, J. C.
Cannabis and Cannabinoid Research. (2020).
doi:10.1089/can.2019.0099
Introduction: Cannabis sativa produces hundreds of bioactive compounds, including cannabinoids and terpenoids. It has been proposed that cannabinoids act in synergy with terpenoids to produce the entourage effect, a concept used to explain the therapeutic benefits of medicinal cannabis. One molecular explanation for the entourage effect is that the terpenoids augment the actions of cannabinoids at their molecular drug targets in cells. We recently reported that terpenoids commonly found in cannabis do not influence the functional effects of D9-tetrahydrocannabinol (D9-THC) on cannabinoid 1 and cannabinoid 2 receptors. The present study aimed to extend on this research by examining whether terpenoids influence the effects of phytocannabinoids and endocannabinoids on human transient receptor potential ankyrin 1 (hTRPA1) and human transient receptor potential vanilloid 1 (hTRPV1) channels heterologously expressed in mammalian cells.
Materials and Methods: The activity of terpenoids, phytocannabinoids, and endocannabinoids was assessed in inducible HEK Flp-In T-Rex cells transfected with hTRPA1 and hTRPV1 channels, respectively. Real-time changes in intracellular calcium ([Ca]i) were measured using the Calcium 5 dye and a FlexStation 3 plate reader.
Results: a-pinene, b-pinene, b-caryophyllene, linalool, limonene, b-myrcene or a-humulene did not affect [Ca]i in hTRPA1 and hTRPV1 overexpressing cells. Cinnamaldehyde (CA), D9-THC, and 2-arachidonoylglycerol (2-AG) activated TRPA1 receptors with high efficacy and similar potency (EC50s of*10 lM). Capsaicin and anandamide (AEA) activated TRPV1 receptors with an EC50 of 61nM and 4.3 lM, respectively, but TRPV1 showed no response to D9-THC, cannabidiol, and other minor cannabinoids. Terpenoids did not significantly affect the responses of TRPA1 and TRPV1 receptors to submaximal and maximal concentrations of CA and D9-THC or the endocannabinoids AEA and 2-AG.
Discussion: We could not find any evidence that the terpenoids tested here activate TRPA1 and TRPV1 channels or modulate their activation by D9-THC and other agonists, including endocannabinoids
Terpenoids From Cannabis Do Not Mediate an Entourage Effect by Acting at Cannabinoid Receptors.
Finlay, D. B., Sircombe, K. J., Nimick, M., Jones, C., & Glass, M.
Frontiers in Pharmacology, 11.(2020).
doi:10.3389/fphar.2020.00359
The entourage effect was a proposed explanation for biological observations that endocannabinoid ligand activities can be modified by other lipids released from cells at the same time. An increasing volume of anecdotal reports and interest in the plant have provoked research into the activity of minor chemical constituents of the plant—including volatile terpenoids such as myrcene, a- and b- pinene, b-caryophyllene, and limonene. However, to date, no clear interaction has been identified. The current study was designed to determine whether terpenes in the cannabis plant have detectable receptor-mediated activity, or modify the activity of D9 -tetrahydrocannabinol, cannabidiol, or the endocannabinoid 2-arachidonylglycerol at the cannabinoid receptors. In addition, we have utilized a standard radioligand binding paradigm with ability to detect orthosteric and allosteric interactions of test compounds. With the possible exception of a weak interaction of b-caryophyllene with CB2, no data were produced to support the hypothesis that any of the five terpenes tested (either alone or in mixtures) have direct interactions with CB1 or CB2, as the binding of radioligand ([3 H]-CP55,940), D9 - tetrahydrocannabinol, and cannabidiol were unaltered by the presence of terpenes. Similarly, terpene functional effects were also not detected, either alone or in combination with D9 -tetrahydrocannabinol, cannabidiol, or 2-arachidonoylglycerol. This study adds to the evidence that the putative entourage effect cannot be explained by direct effects at CB1 or CB2.
Terpinolene, a component of herbal sage, downregulates AKT1 expression in K562 cells
DOI: 10.3892/ol.2011.491
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3362481/
Protein kinase AKT mediates cell proliferation and survival signals, and also contributes to cancer progression. Increased expression and/or activation of AKT is involved in a variety of human cancers. In cells treated with sage or rosemary extract, mRNA and protein expression levels of AKT1 were reduced compared with those of the control cells 48 h after the herbal treatments. We found that terpinolene, a common component of sage and rosemary, markedly reduced the protein expression of AKT1 in K562 cells and inhibited cell proliferation.
Terpli: A Future Driven by Terpenes Versus Anything Else
Jason S. Lupoi
Terpenes and Testing magazine
https://terpenesandtesting.com/terpl...anything-else/
A talk with Terpli CEO Peter Kasper,
Peter: Terpli interprets batch-specific certificates of analysis to analyze the actual chemistry behind each product via the full cannabinoid and terpene profile. We use an algorithm to analyze the sample, submitted reviews, and research information to interpret what a user might expect from a particular product in their hand.
Terpli has designed a unique and personalized mobile app that offers users specific insights into effects they can anticipate from a cannabis variety based on the terpene profile and what other users have experienced from the plant.
The app allows users to look up a particular product from a represented brand via its product name, orby typing in the batch ID from the packaging label, which is run through an algorithm that curates specific effects and outcomes that a user might experience based on the cannabinoid and terpene profile. We work with brands and retailers to provide discounts that act as incentives for our users to write genuine reviews of the products on the platform and help with the high cost of cannabis goods.
That skunky smell? Blame ‘321 MBT’
Hemp Today April 2021
https://hemptoday.net/that-skunky-smell-blame-321-mbt/
American researchers say they have determined what gives cannabis its skunky smell. Scientists from Indiana-based Byers Scientific and Iowa State University say the compound 3-methyl-2-butene-1-thiol (“321 MBT”) is the main source of the odor, and not cannabis plant terpenes, which have generally been considered to cause the smell. The research team used analytical chemistry, leaf enclosure study and field observation to isolate and identify the aroma’s source. 321 MBT is the same chemical that causes the skunky-like aroma in spoiled beer, the scientists said. Hops, the main ingredient in beer, is also of the Cannabaceae plant family. The research is part of efforts to devise ways to eliminate the smell.
The Cannabis sativa Versus Cannabis indica Debate: An Interview with Ethan Russo, MD
Daniele Piomelli and Ethan B. Russo
Cannabis and Cannabinoid Research Jan 2016
DOI: 10.1089/can.2015.29003.ebr
Dr. Ethan Russo, MD, is a board-certified neurologist, psychopharmacology researcher, and Medical Director of PHYTECS, a biotechnology company researching and developing innovative approaches targeting the human endocannabinoid system. Previously, from 2003 to 2014, he served as Senior Medical Advisor and study physician to GW Pharmaceuticals for three Phase III clinical trials of Sativex for alleviation of cancer pain unresponsive to optimized opioid treatment and studies of Epidiolex for intractable epilepsy. He has held faculty appointments in Pharmaceutical Sciences at the University of Montana, in Medicine at the University of Washington, and as visiting Professor, Chinese Academy of Sciences. He is a past President of the International Cannabinoid Research
Society and former Chairman of the International Association for Cannabinoid Medicines. He serves on the Scientific Advisory Board for the American Botanical Council. He is the author of numerous books, book chapters, and articles on Cannabis, ethnobotany, and herbal medicine. His research interests have included correlations of historical uses of Cannabis with modern pharmacological mechanisms, phytopharmaceutical treatment of migraine and chronic pain, and phytocannabinoid/terpenoid/serotonergic/vanilloid interactions.
The Cannabis Terpenes
Sarana Rose Sommano , Chuda Chittasupho , Warintorn Ruksiriwanich and Pensak Jantrawut
Molecules 2020, 25, 5792;
doi:10.3390/molecules25245792
Terpenes are the primary constituents of essential oils and are responsible for the aroma characteristics of cannabis. Together with the cannabinoids, terpenes illustrate synergic and/or entourage effect and their interactions have only been speculated in for the last few decades.
Hundreds of terpenes are identified that allude to cannabis sensory attributes, contributing largely to the consumer’s experiences and market price. They also enhance many therapeutic benefits, especially as aromatherapy. To shed light on the importance of terpenes in the cannabis industry, the purpose of this review is to morphologically describe sources of cannabis terpenes and to explain the biosynthesis and diversity of terpene profiles in different cannabis chemovars.
The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: a placebo-controlled, double-blind, crossover pilot trial.
Amir Englund, Zerrin Atakan, Aleksandra Kralj, Nigel Tunstall, Robin Murray and Paul Morrison
DOI: 10.1177/0269881115615104
In this small pilot study with healthy infrequent cannabis users, results indicate that the dose of 10mg oral THCV is well tolerated with no serious adverse reactions, and was subjectively not distinguishable from placebo. Furthermore, the lower dose of 1mg iv THC did not produce any significant short-term memory impairment, or psychotic or paranoid reactions. THCV significantly inhibited THC-induced impairment to delayed recall as well as THC-induced increase of heart rate. THCV on its own showed signs towards improved performance on the harder working-memory task, while also producing a slight increase in
anxiety. However, these effects were small and need to be further studied in a larger sample.
RATIONALE:
Cannabis is mostly grown under illegal and unregulated circumstances, which seems to favour a product increasingly high in its main cannabinoid ?-9-tetrahydrocannabinol (THC). ?-9-tetrahydrocannabivarin (THCV) is a relatively untestedcannabinoid which is said to be a cannabinoid receptor neutral antagonist, and may inhibit the effects of THC.
OBJECTIVES:
To explore the safety and tolerability of repeated THCV administration and its effects on symptoms normally induced by THC in a sample of healthy volunteers.
METHODS:
Ten male cannabis users (<25 use occasions) were recruited for this within-subjects, placebo-controlled, double-blind, cross-over pilot study. 10mg oral pure THCV or placebo were administered daily for five days, followed by 1mg intravenous THCon the fifth day.
RESULTS:
THCV was well tolerated and subjectively indistinguishable from placebo. THC did not significantly increase psychotic symptoms, paranoia or impair short-term memory, while still producing significant intoxicating effects. Delayed verbal recall was impaired by THC and only occurred under placebo condition (Z=-2.201, p=0.028), suggesting a protective effect of THCV. THCV also inhibited THC-induced increased heart rate (Z=-2.193, p=0.028). Nine out of ten participants reported THC underTHCV condition (compared to placebo) to be subjectively weaker or less intense (?2=6.4, p=0.011). THCV in combination with THC significantly increased memory intrusions (Z=-2.155, p=0.031).
CONCLUSION:
In this first study of THC and THCV, THCV inhibited some of the well-known effects of THC, while potentiating others. These findings need to be interpreted with caution due to a small sample size and lack of THC-induced psychotomimetic and memory-impairing effect, probably owing to the choice of dose.
FYI, I did THCV/THC trials 15 years ago, unpublished, but we found much the same, THCV is not for recreational users unless they like their THC tuned down. THCV delays THC onset, reduces peak experiences, and maybe lengthens the reduced effects.
-SamS
The essential oil of Cannabis sativa.
Malingre T, Hendriks H, Batterman S, Bos R, Visser J (1975)
Planta Med 28: 56–61
DOI: 10.1055/s-0028-1097829
In previous reports the presence of cannabinoids in the distilled essential oil of Cannabis sativa L. was proved, besides the presence of mono- and sesquiterpene hydrocarbons. In this paper the localization of the cannabinoids in the hairs of the leaves and with that the possible biogenetic relation with the components of the essential oil are demonstrated by microscopic examination after colouring tests and gaschromatographic analysis of the isolated contents of individual glandular hairs. Quantitative data about the relation between essential oil and cannabinoids are obtained by comparing the extracts without and after preceding steam distillation. On acount of the origin of the seed (birdseed), special attention was paid to the botanical description of the plant material and to the counting of chromosomes.
NOT CANNABIS SPECIFIC
The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom.
Chen F, Tholl D, Bohlmann J, Pichersky E.
Plant J. 2011 Apr;66(1):212-29.
doi: 10.1111/j.1365-313X.2011.04520.x.
Some plant terpenes such as sterols and carotenes are part of primary metabolism and found essentially in all plants. However, the majority of the terpenes found in plants are classified as 'secondary' compounds, those chemicals whose synthesis has evolved in plants as a result of selection for increased fitness via better adaptation to the local ecological niche of each species. Thousands of such terpenes have been found in the plant kingdom, but each species is capable of synthesizing only a small fraction of this total. In plants, a family of terpene synthases (TPSs) is responsible for the synthesis of the various terpene molecules from two isomeric 5-carbon precursor 'building blocks', leading to 5-carbon isoprene, 10-carbon monoterpenes, 15-carbon sesquiterpenes and 20-carbon diterpenes. The bryophyte Physcomitrella patens has a single TPS gene, copalyl synthase/kaurene synthase (CPS/KS), encoding a bifunctional enzyme producing ent-kaurene, which is a precursor of gibberellins. The genome of the lycophyte Selaginella moellendorffii contains 18 TPS genes, and the genomes of some model angiosperms and gymnosperms contain 40-152 TPS genes, not all of them functional and most of the functional ones having lost activity in either the CPS- or KS-type domains. TPS genes are generally divided into seven clades, with some plant lineages having a majority of their TPS genes in one or two clades, indicating lineage-specific expansion of specific types of genes. Evolutionary plasticity is evident in the TPS family, with closely related enzymes differing in their product profiles, subcellular localization, or the in planta substrates they use.
THE INFLUENCE OF TERPENES ON THE RELEASE OF VOLATILE ORGANIC COMPOUNDS AND ACTIVE INGREDIENTS TO CANNABIS VAPING AEROSOLS
Jiries Meehan-Atrash, Wentai Luo, Kevin J. McWhirter David G. Dennis, David Sarlah, Robert P. Jensen, Isaac Afreh, Jia Jiang, Kelley Barsanti, Alisha Ortiz, Robert M. Strongin
RSC Adv., 2021, 11,11714-11723
DOI: 10.1039/D1RA00934F
Dabbing and vaping cannabis extracts have gained large popularity in the United States as alternatives to cannabis smoking, but diversity in both available products and consumption habits make it difficult to assess consumer exposure to psychoactive ingredients and potentially harmful components. This work studies the how relative ratios of the two primary components of cannabis extracts, D9-tetrahydrocannabinol (THC) and terpenes, affect dosage of these and exposure to harmful or potentially harmful components (HPHCs). THC contains a monoterpene moiety and has been previously shown to emit similar volatile degradation products to terpenes when vaporized. Herein, the major thermal degradation mechanisms for THC and b-myrcene are elucidated via analysis of their aerosol gas phase
products using automated thermal desorption-gas chromatography-mass spectrometry with the aid of isotopic labelling and chemical mechanism modelling. Four abundant products – isoprene, 2-methyl-2-butene, 3-methylcrotonaldehyde, and 3-methyl-1-butene – are shown to derive from a common radical intermediate for both THC and b-myrcene and these products comprise 18–30% of the aerosol gas phase. The relative levels of these four products are highly correlated with applied power to the ecigarette, which indicates formation of these products is temperature dependent. Vaping THC–bmyrcene mixtures with increasing % mass of b-myrcene is correlated with less degradation of the starting material and a product distribution suggestive of a lower aerosolization temperature. By contrast, dabbing THC–b-myrcene mixtures with increasing % mass of b-myrcene is associated with higher levels of HPHCs, and isotopic labelling showed this is due to increased reactivity of b-myrcene relative to THC.
Not Cannabis specific
The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers
Natalia Dudareva, Susanna Andersson, Irina Orlova, Nathalie Gatto, Michael Reichelt, David Rhodes, Wilhelm Boland, and Jonathan Gershenzon
Proc Natl Acad Sci U S A. Jan 18, 2005; 102(3): 933–938.
doi: 10.1073/pnas.0407360102
Terpenoids, the largest class of plant secondary metabolites, play essential roles in both plant and human life. In higher plants, the five-carbon building blocks of all terpenoids, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate, are derived from two independent pathways localized in different cellular compartments. The methylerythritol phosphate (MEP or nonmevalonate) pathway, localized in the plastids, is thought to provide IPP and dimethylallyl diphosphate for hemiterpene, monoterpene, and diterpene biosynthesis, whereas the cytosol-localized mevalonate pathway provides C5 units for sesquiterpene biosynthesis. Stable isotope-labeled, pathway-specific precursors (1-deoxy-[5,5-2H2]-d-xylulose and [2,2-2H2]-mevalolactone) were supplied to cut snapdragon flowers, which emit both monoterpenes and the sesquiterpene, nerolidol. We show that only one of the two pathways, the plastid-localized MEP pathway, is active in the formation of volatile terpenes. The MEP pathway provides IPP precursors for both plastidial monoterpene and cytosolic sesquiterpene biosynthesis in the epidermis of snapdragon petals. The trafficking of IPP occurs unidirectionally from the plastids to cytosol. The MEP pathway operates in a rhythmic manner controlled by the circadian clock, which determines the rhythmicity of terpenoid emission
The Monoterpene Ocimene: Characteristics, Sources, and Benefits
Derek Johnson
Terpenes and Testing magazine
https://terpenesandtesting.com/the-m...-and-benefits/
Ocimene is an isomeric hydrocarbon as well as a monoterpene. When exposed to air, it becomes extremely volatile, which helps explain its highly aromatic characteristics. Ocimene occurs as three different isomers: α-ocimene, cis-β-ocimene, and trans-β-ocimene.
Ocimene is highly valued for its aromatic profile. It contributes to the strong and pleasant aromas of many fruits, herbs, and spices, including tarragon, pepper, basil, mango, mint, and parsley. The odor has been described as herbaceous, citrusy, tropical, and woody.
Ocimene is also present in cannabis. One popular dispensary cites cultivars Jack Herer and Mandarin Dream. Another adds Sour Diesel, Clementine, and Snowcone to the list. It is also a key terpene in the medical cultivar Bedrocan.
Ocimene is associated with anti-fungal, anticonvulsant, and anti-tumor activities. It has also been reported to play an important role as a pheromone for the social regulation of honeybees.
One study focused on essential oil from the Angelica plant, which contains principally α-pinene and cis-β-ocimene. The oil and the two isolated terpenes proved effective against Candida albicans, Cryptococcus neoformans, and dermatophytes (skin fungus).
The next evolution in Horticulture lighting –Impact of Using Supplemental UV
Peter Barber
https://issuu.com/amsterdamrai/docs/peter_barber.pptx
Shows that a very narrow band of UVB can help increase Terpenes and Cannabinoids as well as help control PM and Botrytris on Cannabis crops
The preservation and augmentation of volatile terpenes in cannabis inflorescence
Justin Bueno, Emily Leuer, Michael Kearney Jr, Edward H. Green and Eric A. Greenbaum
Journal of Cannabis Research (2020) 2:27
DOI: 10.1186/s42238-020-00035-z
Terpenes contribute to the pharmacology, efficacy, aroma, and flavor of cannabis inflorescence, improving the experience for medical and recreational users. Terpenes are inherently volatile, resulting in the loss of terpene content as inflorescence ages. A method to establish and/or maintain a desired terpene content of cannabis inflorescence is needed. A novel packaging method was investigated for the preservation of native terpenes and the replenishment of terpenes to depleted inflorescence over various storage durations. Methods: Inflorescence samples from two different chemotypes (DJ’s Gold, Cream Caramel) were obtained from a state licensed medical cannabis organization. Samples from the DJ’s Gold chemotype were depleted of terpenes whereas samples from the Cream Caramel chemotype had a terpene content representative of inflorescence available for medicinal or recreational purposes. Inflorescence samples were stored using the novel packaging approach, in airtight containers in the presence of external terpenes. Control samples were similarly stored without external terpenes. Terpene content of the inflorescence samples were quantitively determined by headspace gas chromatography mass spectrometry (HS GC-MS) after various storage durations. Main effects analysis was used to determine the impact of various parameters on the effectiveness of the system. Results: All samples stored using the novel packaging approach had a higher terpene content than their corresponding control. 1.18% (w/w) of external terpene, relative to inflorescence weight, was the minimum amount required to maintain the initial terpene content of the inflorescence after 6 weeks of storage. Main effects analysis showed that augmentation of inflorescence terpene content was dependent upon the amount and type of external volatile utilized. The terpene profile of inflorescence samples from two separate harvests were selectively adjusted, reducing the percent difference of the two sample’s terpene profiles by 39.5%. Conclusions: A successful proof of concept was achieved for preservation, augmentation, and replenishment of terpenes to cannabis inflorescence over various storage durations. Inflorescence stored using the novel packaging approach is a significant step towards providing patients with cannabis inflorescence of reproducible and reliable terpene content, an important component of inflorescence efficacy. The novel approach for replenishment of terpenes to depleted inflorescence represents an exciting development for patients and manufacturers.
The Problem of Concentrate Consistency: Terpene Loss in Extraction
Steep Hill
https://static1.squarespace.com/stat...Terpenes_2.pdf
Cannabis Extraction Methods Alter Terpene Concentrations
The modern cannabis consumer doesn’t rely solely on whole-flower methods of consumption. Many modern cannabis consumers—whether medical cannabis patients or recreational consumers—have embraced the convenience, discretion, and rapid relief offered by personal vaping pens or concentrate rigs, which offer consistent, convenient ways to dose discreetly. The question, then, is whether consumers who prefer to use cannabis extracts as opposed to whole-flower use are getting the consistency of experience that they need, as well as the certainty that the product they’re purchasing lives up to the chemovar profile of the strain used in its labeling and marketing.
THE PRODUCTION OF ESSENTIAL HEMP OIL IN SWITZERLAND
Vito Mediavilla
https://www.researchgate.net/publica...IN_SWITZERLAND
Essential hemp oil is an interesting product, which may be obtained from hemp. This concentrate of the typical hemp aroma is utilised in cosmetics, additives to food, aroma therapy and perfume. Since 1995 a couple of Swiss farmers produced with very high labour input high quality oils. Their sale
potential is not clear yet and at present it is a niche product. Hemp regulations in some countries (e.g. USA) limit its import. We assessed the factors influencing yield and quality in some experiments. Recommendations about variety choice, harvest time, weather influence, seed density, harvest
techniques and prevention of pollination in function to yield and smell scent are discussed. For a broader propagation of essential hemp oil additional information about medical (pharmaceutical and dermatological) use is needed.
Not cannabis specific
The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections.
Cappiello, F., Loffredo, M. R., Del Plato, C., Cammarone, S., Casciaro, B., Quaglio, D., … Ghirga, F.
Antibiotics, 9(6), 325.(2020).
doi:10.3390/antibiotics9060325
he discovery of antibiotics has revolutionized the medicine and treatment of microbial infections. However, the current scenario has highlighted the difficulties in marketing new antibiotics and an exponential increase in the appearance of resistant strains. On the other hand, research in the field of drug-discovery has revaluated the potential of natural products as a unique source for new biologically active molecules and scaffolds for the medicinal chemistry. In this review, we first contextualized the worldwide problem of antibiotic resistance and the importance that natural products of plant origin acquire as a source of new lead compounds. We then focused on terpenes and their potential development as antimicrobials, highlighting those studies that showed an activity against conventional antibiotic-resistant strains
Not Cannabis specific
The role of structure and molecular properties of terpenoids in determining their antimicrobial activity.
Griffin, S. G., Wyllie, S. G., Markham, J. L., & Leach, D. N.
Flavour and Fragrance Journal, 14(5), 322–332.(1999).
doi:10.1002/(sici)1099-1026(199909/10)14:5<322::aid-ffj837>3.0.co;2-4
The minimum inhibitory concentrations (MIC) of 60 terpenoids against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and Candida albicans have been determined. Hierarchical cluster analysis was used to group the compounds into ®ve groups according to their activity patterns against the four microorganisms. K-Means cluster analysis was then used to con®rm these groupings and to show the dierences in the activity patterns of the groups. Ten molecular properties of the terpenoids, either calculated via molecular modelling or determined by direct measurement, were then used as variables in a forward stepwise discriminant analysis to identify which variables discriminated between groups. Low water solubility of Group IV compounds, mainly hydrocarbons and acetates, was found to be associated with their relative inactivity. The remaining groups, all containing oxygenated terpenoids, showed characteristic but distinct activity patterns towards the four test organisms. Hydrogen bonding parameters were found to be associated with antimicrobial activity in all cases. Activity against Gram-negative E. coli and P. aeruginosa was associated with a combination of a hydrogen bonding and size parameters. This was not found to be the case for the Gram-positive S. aureus or the yeast C. albicans.
The sedative effect of inhaled terpinolene in mice and its structure–activity relationships.
Ken Ito, Michiho Ito
Journal of Natural Medicines 67(4) January 2013
DOI: 10.1007/s11418-012-0732-1
Terpinolene is a cyclic monoterpene compound found in some Labiatae herbs. In our previous study, we evaluated the sedative effect of inhaled essential oils of Microtoena patchoulii leaves in mice and isolated terpinolene as an active ingredient. We investigated the structure-activity relationships of terpinolene to identify the structural part essential to its sedative effect. Comparison of terpinolene analog activities showed that a double bond in the side-chain or pi bonds in the six-membered ring play important roles in the sedative effect. In another experiment using olfactory impaired mice, we further revealed that inhaled terpinolene exerted the effect after nasal absorption into the body.
The Terroir of Cannabis: Terpene Metabolomics as a Tool to Understand Cannabis sativa Selections.
Mudge, E., Brown, P., & Murch, S. (2019).
Planta Medica.
doi:10.1055/a-0915-2550
The phytochemical diversity of Cannabis chemovars is not well understood, and many chemovars were created in informal breeding programs without records of parentage or the criteria for selection. Key criteria for selection sometimes included aroma notes and visual cues, which some breeders associated with pharmacological activity. We hypothesized that the process of selection for scents believed to be related to specific tetrahydrocannabinol levels has resulted in modified terpene biosynthesis in these chemovars. Thirty-two cannabinoids, 29 monoterpenes and 38 sesquiterpenes were measured in 33 chemovars from 5 licensed producers. A classification system based on cannabinoid content was used with targeted metabolomic tools to determine relationships in the phytochemistry. Three monoterpenes, limonene, ?-myrcene, and ?-pinene, and two sesquiterpenes, caryophyllene and humulene, were abundant in the majority of chemovars. Nine terpenes were present in tetrahydrocannabinol-dominant chemovars. Three monoterpenes and four sesquiterpenes were predominantly found in cannabidiol-containing chemovars. Low abundance terpenes may have been the aromatic cues identified by breeders. The medicinal activity of some of the terpenes is likely to contribute to the pharmacological effect of specific chemovars. Together, these data demonstrate the synergy of compounds in Cannabis chemovars and point to the need for additional research to understand the phytochemical complexity.
The Volatile Oil Composition of Fresh and Air-Dried Buds of Cannabis sativa
Samir A. Rossi and Mahmoud A. ElSohly
J. Nut. Prod. 1996, 59, 49-51
DOI: 10.1021/np960004a
The composition of the steam-distilled volatile oil of fresh and air-dried, indoor-grown marijuana was studied by GC/FID and GC/MS. In all, 68 components were detected of which 57 were fully identified. Drying of the plant material had no effect on the qualitative composition of the oil and did not affect the ability of individuals familiar with marijuana smell to recognize the odor
Therapeutic and Medicinal Uses of Terpenes
Destinney Cox-Georgian, Niveditha Ramadoss, Chathu Dona, and Chhandak Basu
Book, Medicinal Plants, N. Joshee et al. (eds.), Chapter 15
DOI: 10.1007/978-3-030-31269-5
DOI: 10.1007%2F978-3-030-31269-5_15
Terpenes, also known as terpenoids are the largest and most diverse group of naturally occurring compounds. Based on the number of isoprene units they have, they are classified as mono, di, tri, tetra, and sesquiterpenes. They are mostly found in plants and form the major constituent of essential oils from plants. Among the natural products that provide medical benefits for an organism, terpenes play a major and variety of roles. The common plant sources of terpenes are tea, thyme, cannabis, Spanish sage, and citrus fruits (e.g., lemon, orange, mandarin). Terpenes have a wide range of medicinal uses among which antiplasmodial activity is notable as its mechanism of action is similar to the popular antimalarial drug in use—chloroquine. Monoterpenes specifically are widely studied for their antiviral property. With growing incidents of cancer and diabetes in modern world, terpenes also have the potential to serve as anticancer and antidiabetic reagents. Along with these properties, terpenes also allow for flexibility in route of administration and suppression of side effects. Certain terpenes were widely used in natural folk medicine. One such terpene is curcumin which holds anti-inflammatory, antioxidant, anticancer, antiseptic, antiplasmodial, astringent, digestive, diuretic, and many other properties. Curcumin has also become a recent trend in healthy foods and open doors for several medical researches. This chapter summarizes the various terpenes, their sources, medicinal properties, mechanism of action, and the recent studies that are underway for designing terpenes as a lead molecule in the modern medicine
Thermal Degradation of Terpenes: Camphene, A3-Carene, Limonene, and ot=Terpinene
GERALD W. MCGRAW, RICHARD W. HEMINGWAY, LEONARD L. INGRAM, JR., CATHERINE S. CANADY, AND WILLIAM B. MCGRAW
VOL. 33, NO. 22,1999 I ENVIRONMENTAL SCIENCE & TECHNOLOGY
https://www.srs.fs.fed.us/pubs/ja/ja_mcgraw001.pdf
Emissions from wood dryers have been of some concern for a number of years, and recent policy changes by the Environmental Protection Agency have placed emphasis upon the gaseous emissions that lead to the formation of particulate matter as small as 2.5 pm diameter. In this qualitative study, camphene, AQarene, limonene, and a-terpinene were thermally degraded in the presence of airto determine the number and kind of oxidative degradation products that might be expected under drying conditions used in processing wood productsvarious chromatographic methods were used to isolate the products for proof of structure by NMR and/or GC-MS.The degradation products resulted from dehydrogenations, epoxidations, double bond cleavages, allylic oxidations, and rearrangements. A number of compounds not previously associated with the thermal degradation of these terpenes were identified
Tingenone, a pentacyclic triterpene, induces peripheral antinociception due to cannabinoid receptors activation in mice
C. C. Veloso, R. C. M. Ferreira, V. G. Rodrigues, L. P. Duarte, A. Klein
I. D. Duarte, T. R. L. Romero, A. C. Perez
Inflammopharmacol
DOI 10.1007/s10787-017-0391-7
Several works have shown that triterpenes induce peripheral antinociception by activation of cannabinoid receptors and endocannabinoids; besides, several research groups have reported activation of cannabinoid receptors in peripheral antinociception. The aim of this study was to assess the involvement of the cannabinoid system in the antinociceptive effect induced
by tingenone against hyperalgesia evoked by prostaglandin E2 (PGE2 ) at peripheral level. The paw pressure test was used and the hyperalgesia was induced by intraplantar injection of PGE2 (2 l g/paw). All drugs were injected subcutaneously in the hind paws of male Swiss mice. Tingenone (200 l g/paw) administered into the right hind paw induced a local antinociceptive effect, that was antagonized by AM630, a selective antagonist to CB2 cannabinoid receptor. AM251, a selective antagonist to CB1 cannabinoid receptor, did not alter the peripheral antinociceptive effect of tingenone. MAFP, a fatty acid amide hydrolase (FAAH) inhibitor; VDM11, an anandamide reuptake inhibitor; and JZL184, monoacylglycerol lipase (MAGL) inhibitor did not potentiate the peripheral antinociceptive effect of the lower dose of tingenone (50 l g/paw). The results suggest that tingenone induced a peripheral antinociceptive effect via cannabinoid receptor activation. Therefore, this study suggests a pharmacological potential for a new analgesic drug.
Toxicant Formation in Dabbing: The Terpene Story
Jiries Meehan-Atrash, Wentai Luo, Robert M Strongin
Project: Cannabis vaporizing September 2017 DOI: 10.1021/acsomega.7b01130
Inhalable, noncombustible cannabis products are playing a central role in the expansion of the medical and recreational use of cannabis. In particular, the practice of “dabbing” with butane hash oil has emerged with great popularity in states that have legalized cannabis. Despite their growing popularity, the degradation product profiles of these new products have not been extensively investigated. The study herein focuses on the chemistry of myrcene and other common terpenes found in cannabis extracts. Methacrolein, benzene, and several other products of concern to human health were formed under the conditions that simulated real-world dabbing. The terpene degradation products observed are consistent with those reported in the atmospheric chemistry literature.
Not Cannabis specific
Traversing the fungal terpenome
Maureen B. Quin, Christopher M. Flynn and Claudia Schmidt-Dannert
Nat. Prod. Rep., 2014, 31, 1449
DOI: 10.1039/c4np00075g
Fungi (Ascomycota and Basidiomycota) are prolific producers of structurally diverse terpenoid compounds. Classes of terpenoids identified in fungi include the sesqui-, di- and triterpenoids. Biosynthetic pathways and enzymes to terpenoids from each of these classes have been described. These typically involve the scaffold generating terpene synthases and cyclases, and scaffold tailoring enzymes such as e.g. cytochrome P450 monoxygenases, NAD(P)+ and flavin dependent oxidoreductases, and various group transferases that generate the final bioactive structures. The biosynthesis of several sesquiterpenoid mycotoxins and bioactive diterpenoids has been well-studied in Ascomycota (e.g. filamentous fungi). Little is known about the terpenoid biosynthetic pathways in Basidiomycota (e.g. mushroom forming fungi), although they produce a huge diversity of terpenoid natural products. Specifically, many trans-humulyl cation derived sesquiterpenoid natural products with potent bioactivities have been isolated. Biosynthetic gene clusters responsible for the production of transhumulylcation derived protoilludanes, and other sesquiterpenoids, can be rapidly identified by genome sequencing and bioinformatic methods. Genome mining combined with heterologous biosynthetic pathway refactoring has the potential to facilitate discovery and production of pharmaceutically relevant fungal terpenoids.
Ultrasound-assisted extraction of volatile compounds from industrial Cannabis sativa L. inflorescences
Da Porto C, Decorti D, Natolino A
International Journal of Applied Research in Natural Products
2014 Vol. 7 (1), pp. 8-14
doi: 10.1111/1750-3841.14075
This study investigated the use of ultrasound-assisted extraction (UAE) to recovery volatile compounds from the inflorescences of a fiber type Cannabis sativa L. cultivar. The results show that ultrasonic treatment not longer than 5 min allows to obtain an enhanced concentration of terpenes in comparison with maceration. Instead, an ultrasonic treatment longer than 5 min increased the concentration of ?-9-tetraidrocannabinol (THC). A preliminary screening of cannabis inflorescences scent was performed by headspace solid-phase microextraction (HS-SPME) with gas chromatography-mass spectrometry (GC-MS) avoiding the chemical modification and artifact formation that can occur in conventional methods.
Use of ABA/Methyl Jasmonate/Sugar to increase terpene and cannabinoid production in cannabis sativa
https://www.patentguru.com/US10874102B2
https://www.patentguru.com/US2018279611A1
https://patents.google.com/patent/US20180279611A1
The invention involves the use of a combination of s-abscisic acid (S-ABA), methyl jasmonate (MJ), glucose, sucrose and fructose to raise the level of cannabinoids and terpenes (flavor and scent molecules) in the Cannabis Sativa plant.
*UVB lights to increase terpenes and secondary metabolites
Peter Barber
GreenTech Amsterdam Rai 2018
https://issuu.com/amsterdamrai/docs/peter_barber.pptx
Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods.
Namdar, D., Mazuz, M., Ion, A., & Koltai, H.
Industrial Crops and Products, 113, 376–382.(2018).
doi:10.1016/j.indcrop.2018.01.060
In the last decade, recognition of the therapeutic abilities of Cannabis sativa has risen, along with the need to standardize its products. Standardization requires grading the methods for growing the plant and extracting the active compounds accumulated in its inflorescence. We explored the results of different methods used today and their effect on the levels of compounds extracted from inflorescences positioned along the C. sativa flowering stem. The polarity of the solvent used for the extraction, drying processes and separation methods influenced the chemical composition of the extract. However, regardless of extraction and analytical methods applied, the amounts of cannabinoids and terpenoids in the inflorescences decreased with the position of the sampled in- florescence from top to bottom of the flowering stem. These results have significant implications for the development of growth protocols for C. sativa cultivation and flower extraction methods to standardize cannabisbased products
Variations in Terpene Profiles of Different Strains of Cannabis sativa L
Salvatore Casano, Gianpaolo Grassi, V Martini, Marco Michelozzi
December 2011 Acta horticulturae 925(925):115-121
DOI: 10.17660/ActaHortic.2011.925.15
Secondary compounds of the plant are indispensable to cope with its often hostile environment and the great chemical diversity and variability of intraspecific and interspecific secondary metabolism is the result of natural selection. Recognition of the biological properties of secondary compounds have increased their great utility for human uses; numerous compounds now are receiving particular attention from the pharmaceutical industry and are important sources of a wide variety of commercially useful base products. Medical and other effects of Cannabis sativa L. are due to concentration and balance of various active secondary metabolites, particularly the cannabinoids, but including also a wide range of terpenoids and flavonoids. A wide qualitative and quantitative variability in cannabinoids, terpenoids, and flavonoids contents in Cannabis species are apparent from reports in the literature. Terpenes are strongly inherited and little influenced by environmental factors and, therefore, have been widely used as biochemical marker in chemosystematic studies to characterize plant species, provenances, clones, and hybrids. This study investigated the variability of terpene profiles in C. sativa. The terpene composition in inflorescences of samples collected from progenies of 16 plants derived from different strains was analysed by GC/FID. The amount of each terpene (in sufficient quantities to be considered in statistical analysis) was expressed as a percentage of total terpenes. Results showed a large variation between different strains in the relative contents for several monoterpenes (?-pinene, camphene, ?-pinene, sabinene, ?-3-carene, ?-phellandrene, ?-myrcene, ?-terpinene, limonene, 1.8-cineole, ?-terpinene, cis-?-ocimene, trans-?- ocimene, ?-terpinolene) and one sesquiterpene, ?-caryophyllene. This variability in terpene composition can provide a potential tool for the characterization of Cannabis biotypes and warrant further research to evaluate the drug’s medical value and, at the same time, to select less susceptible chemotypes to the attack of herbivores and diseases.
Part 2, Which Terpenes Are in ‘Haze’ Strains?
WILL HYDE
https://www.leafly.ca/news/strains-p...-strain-family
In this series, Leafly explores what makes each family of strains unique based on their terpene profiles. A strain “family” refers to a line of hybrids branching from one genetic matriarch that expresses unique and desirable characteristics that breeders seek to build upon. This introductory primer will help you learn a little more about cannabis breeding and strain variability
Not Cannabis specific
The role of structure and molecular properties of terpenoids in determining their antimicrobial activity.
Griffin, S. G., Wyllie, S. G., Markham, J. L., & Leach, D. N.
Flavour and Fragrance Journal, 14(5), 322–332.(1999).
doi:10.1002/(sici)1099-1026(199909/10)14:5<322::aid-ffj837>3.0.co;2-4
The minimum inhibitory concentrations (MIC) of 60 terpenoids against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and Candida albicans have been determined. Hierarchical cluster analysis was used to group the compounds into ®ve groups according to their activity patterns against the four microorganisms. K-Means cluster analysis was then used to con®rm these groupings and to show the dierences in the activity patterns of the groups. Ten molecular properties of the terpenoids, either calculated via molecular modelling or determined by direct measurement, were then used as variables in a forward stepwise discriminant analysis to identify which variables discriminated between groups. Low water solubility of Group IV compounds, mainly hydrocarbons and acetates, was found to be associated with their relative inactivity. The remaining groups, all containing oxygenated terpenoids, showed characteristic but distinct activity patterns towards the four test organisms. Hydrogen bonding parameters were found to be associated with antimicrobial activity in all cases. Activity against Gram-negative E. coli and P. aeruginosa was associated with a combination of a hydrogen bonding and size parameters. This was not found to be the case for the Gram-positive S. aureus or the yeast C. albicans.
The sedative effect of inhaled terpinolene in mice and its structure–activity relationships.
Ken Ito, Michiho Ito
Journal of Natural Medicines 67(4) January 2013
DOI: 10.1007/s11418-012-0732-1
Terpinolene is a cyclic monoterpene compound found in some Labiatae herbs. In our previous study, we evaluated the sedative effect of inhaled essential oils of Microtoena patchoulii leaves in mice and isolated terpinolene as an active ingredient. We investigated the structure-activity relationships of terpinolene to identify the structural part essential to its sedative effect. Comparison of terpinolene analog activities showed that a double bond in the side-chain or pi bonds in the six-membered ring play important roles in the sedative effect. In another experiment using olfactory impaired mice, we further revealed that inhaled terpinolene exerted the effect after nasal absorption into the body.
Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation.
Fiorini, Dennis; Molle, Arnaud; Nabissi, Massimo; Santini, Giuseppe; Benelli, Giovanni; Maggi, Filippo
Industrial Crops and Products, 128(), 581–589. (2019).
doi:10.1016/j.indcrop.2018.10.045
By products of industrial hemp (Cannabis sativa L.), including inflorescences, represent an exploitable material to produce niche products for the pharmaceutical, nutraceutical, cosmetic and pesticide industry. One of them is the essential oil, whose composition can be properly modulated on an industrial level by optimizing the extractive conditions and sample pretreatment. This allows to achieve high concentrations of bioactive compounds, such as cannabidiol (CBD) and sesquiterpenes [e.g. (E)-caryophyllene]. In the present work, we evaluated the effects of type of distillation apparatus, status of conservation of the plant material, grinding and sample-pretreatment with microwave and heat, on the hemp essential oil chemical profile obtained from the monoecious cultivar Felina 32. Seven marker compounds, including the monoterpenes α-pinene, myrcene and terpinolene, the sesquiterpenes (E)-caryophyllene, α-humulene and caryophyllene oxide, and the cannabinoid CBD were quantified in the different hemp essential oil samples by gas chromatography-flame ionization detection (GC-FID) analysis, whereas the overall chemical profiles were achieved by gas chromatography-mass spectrometry (GC–MS) analysis. Results showed that hydrodistillation (HD) in comparison with steam distillation (SD) gave a higher content of cannabinoids. Drying was fundamental to induce decarboxylation of cannabinoid acids to the relative alcoholic forms, coupled with an increase of the sesquiterpene fraction. The optimization of sample pretreatments pointed out that the exposure of dry inflorescences to microwave heating at 900 W power for 1 min was the best method to increase the abundance of bioactive compounds in the essential oil, with special reference to CBD, (E)-caryophyllene and caryophyllene oxide. Overall, these results give new insights into the exploitation of hemp byproducts in different fields such as pharmaceuticals, nutraceuticals and eco-friendly insecticides.
Vapor Phase Terpenes Mitigate Oxidative Degradation of Cannabis sativa Inflorescence Cannabinoid Content in an Accelerated Stability Study
Justin Bueno, Solmaz Alborzi, and Eric A. Greenbaum Cannabis and Cannabinoid Research Volume X, Number X, 2022
DOI: 10.1089/can.2021.0207
Introduction: As Cannabis sativa L. (Cannabaceae) ages, inflorescence phytochemicals are susceptible to oxidative degradation. Reduction of D9-tetrahydrocannabinol (D9-THC) content has the potential to impact the reliability and accuracy of dosing. Advances that improve cannabinoid stability during storage would have an important impact in medical cannabis markets. Reported here is the use of C. sativa terpenes with antioxidant properties that improve inflorescence cannabinoid stability.
Materials and Methods: Killer Kush inflorescence samples were stored in a temperature-controlled environment, in opaque jars. To accelerate the rate of oxidate degradation, samples were stored with the oxidizing agent hydrogen peroxide. Vapor phase terpenes were added to inflorescence packaging. Two terpene blends and three different dosage amounts were evaluated. Inflorescence stability samples were prepared in triplicate for each sample type. Cannabinoid content was quantitatively assessed after 24, 81, and 127 days of storage using high-performance liquid chromatography. Terpene content was assessed using headspace gas chromatography mass spectrometry. Results from inflorescence stored with and without external terpenes were compared by analysis of variance (ANOVA) data processing.
Results: After 127 days of storage, inflorescence in the accelerated study experienced a loss of 18.0% and 34.3% total D9-THC content for samples stored with and without external terpenes, respectively. The differences in cannabinoid content were found to be statistically significant at all time points using ANOVA processing. In the non accelerated study, only one of the six sample types investigated had a statistically significant greater total D9-THC content than control at all time points. Nevertheless, a dose-dependent relationship between the amount of external terpenes added to inflorescence and the preservation of total D9-THC content was observed.
Discussion: In the accelerated study, exogenous terpenes reduced the degradation of inflorescence cannabinoid content by 47.4%. This represents the first reported addition of terpene antioxidants to inflorescence packaging for cannabinoid preservation. Of note, the antioxidants used in this system can be obtained from C. sativa. This is advantageous from a toxicological perspective as inhaling synthetic antioxidants presents unknown and unpredictable risks. When fully developed, the novel system has applications for inflorescence packaged for individual sale, as well as long-term storage of bulk biomass.
Find Pdf
Verdant Whisperer
Well-known member
A thought i had looking through that a 2nd time - Plants use the elements in their environment as efficiency as possible, for example a plant that is high in sulfur compounds may come from a sulfur rich environment and has evolved to use the tools it has available to combat for itself against fungus and pest, while a plant from an area rich in resources yet low in sulfur, would most likely have evolved to use its terpenes, or tannins to combat pest more efficiently than trying to make sulfur compounds without high availability of sulfur… some of the skunky strains have more sulfur compounds in them, the article states a specific name but that’s what they are. I'm sure all plants in a sense evolved around their nutrient availability and native environments, as well as evolved their defenses around them. I think its cool how potent some of the indoor strains are, but there is something to growing landraces where there from will have the most potential. one practice to limit shock from crops non-native is to create and use F1 Hybrids introducing increased vigor allowing it to thrive in foreign environments.
"nutrient niche specialization." This concept suggests that plants have evolved specific adaptations to utilize the nutrients that are most abundant in their native environments. This adaptation extends to how they defend themselves against pests and pathogens.
"nutrient niche specialization." This concept suggests that plants have evolved specific adaptations to utilize the nutrients that are most abundant in their native environments. This adaptation extends to how they defend themselves against pests and pathogens.
Last edited:
Robert o Connel ClarkeAny chance that you could provide source info on the claim that THC alone will not get one high ?
its a defferent delivery system , smoke go's thru lungs in to blood stream , consuming , stomach acids digest in and then its absorbed thru the kindeys , why if a differnt highSomething I've wondered is if terpenes are mainly responsible for the high, what about edibles? I consume cannabis by vaping, eating both fermented (cobs) and decarbed bud, mixed with various oils. On any given variety the highs are similar, although not identical. Anyone familiar with cobs would know that the taste is completely transformed, and I assume so are the terpenes? So why does the high remain essentially the same?
Brother Nature
Well-known member
What I'm reading from your post here is that terpenes have a relational effect to the cannabinoid effect for the smoker, right? This is something that has been studied for quite a while and is pretty much the prevailing thought in the community that studies these types of things. There are indeed many, many articles related to your train of thought in the link I posted. A quick ctrl+f using the word terpene brings up the below quoted article, touching on what you first posted in this thread. I wasn't trying to criticize, but point out that there are many other real people in the world who have the same questions and tested their own theories. I think it is good to learn what is out there to help in forming your own theories. Basing something off results from a search engine geared to learn from and sell shit to you isn't the greatest place to start. There is some of the best cannabis information available on this site alone, you just have to learn how to find it.Not one article in that whole page even closely related to this thead, this thread and alot ive started are creative and new perspectives.
Beta Caryophyllene - A Terpene or A Cannabinoid?
Noel Palmer, Ph.D., Chief Scientist, CBx Sciences
Most people understand that cannabis is responsible for producing cannabinoids; most notably delta-9 THC and CBD. What many people don’t fully understand is that cannabis produces other phytochemicals (chemicals produced by plants) that are therapeutically active. Terpenes are a class of phytochemicals produced by cannabis (and other plants), and in fact scientists believe that terpenes serve as the building blocks for cannabinoids in the cannabis plant. That being said, terpenes in and of themselves are considered to be therapeutically relevant in many ways.
Terpenes are naturally occurring compounds produced by plants and insects that are characterized by their aromatic properties. Many people believe that terpenes are responsible for the flavor and smell of different varieties of cannabis. In this article, we are going to talk about beta-caryophyllene (BCP), a sesquiterpene produced in high proportions in cannabis. Some research suggests that beta-caryophyllene is the most common terpene found in cannabis, and also is regularly found in the public food supply from a variety of sources (e.g. black pepper) . BCP is also found in the essential oils of a variety of plants, including rosemary, hops and cloves, and is even an FDA-approved food additive.
The therapeutic activity of beta-caryophyllene can be partially described as a CB2 agonist – suggesting its role as an anti-inflammatory agent. On top of being a CB2 agonist, beta-caryophyllene has been shown to be active on a number of receptor targets in the body – making it a good candidate for neuropathic pain and neurodegenerative diseases – as well as being an antioxidant agent and antimicrobial compound. The bioavailability and metabolism of beta-caryophyllene still needs to undergo scientific scrutiny. This is especially true in regards to smoking or vaporizing cannabis in which blood levels of beta-caryophyllene (and all terpenes) have yet to be resolved. Obtaining this data will be useful as science tries to understand how terpenes can potentiate or attenuate the effects from both THC and CBD.
Unlike other terpenes, BCP has properties similar to cannabinoids - and some researchers suggest that it may indeed actually be classified as a cannabinoid. While BCP was first synthesized in 1964, in 2008 a group of European scientists led by Andreas Zimmer, Ph.D and Ildiko Racz, Ph.D of the University of Bonn, suggested that BCP is a cannabinoid that acts on CB2 cannabinoid pathways. However, CB1 receptors, the pathways responsible for THC’s effects, are not affected by BCP.
Published in the Journal Proceedings of the National Academy of Sciences (PNAS), the researchers explained:
“Here, we report that the widespread plant volatile β-caryophyllene (BCP) selectively binds to the CB2 receptor and that it is a functional CB2 agonist. Intriguingly, BCP is a common constituent of the essential oils of numerous spice and food plants and a major component in Cannabis.”
Racz and Zimmer’s follow up study, published in 2013 in the Journal European Neuropsychopharmacology, shows that BCP produces significant anti-inflammatory effects. Using mice, the study found that BCP dosed orally was more effective than injections of the synthetic CB2 cannabinoid JWH-133, suggesting that BCP could provide better efficacy than synthetic cannabinoids.
“It is likely that BCP belongs to a group of common plant natural products with major potential impact on human health. The oral intake of this dietary cannabinoid with vegetable food could be advantageous in the daily routine clinical practice over synthetic cannabinoid agonists.”
With its numerous potential benefits, BCP and other terpenes like it are an important part of our understanding of The Entourage Effect -- the theory that a diverse, naturally occurring variety of terpenes and cannabinoids work synergistically to improve health or fight disease. Put simply, the idea suggests that the beneficial impact of the whole plant is greater than the sum of its individual parts. More research will likely uncover additional potential benefits of BCP and other terpenes, and we are excited to be a part of those efforts.
Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects
Ethan B Russo
First published: 12 July 2011
https://doi.org/10.1111/j.1476-5381.2011.01238.x
Citations: 923
Sections
Tools
Share
Abstract
Tetrahydrocannabinol (THC) has been the primary focus of cannabis research since 1964, when Raphael Mechoulam isolated and synthesized it. More recently, the synergistic contributions of cannabidiol to cannabis pharmacology and analgesia have been scientifically demonstrated. Other phytocannabinoids, including tetrahydrocannabivarin, cannabigerol and cannabichromene, exert additional effects of therapeutic interest. Innovative conventional plant breeding has yielded cannabis chemotypes expressing high titres of each component for future study. This review will explore another echelon of phytotherapeutic agents, the cannabis terpenoids: limonene, myrcene, α-pinene, linalool, β-caryophyllene, caryophyllene oxide, nerolidol and phytol. Terpenoids share a precursor with phytocannabinoids, and are all flavour and fragrance components common to human diets that have been designated Generally Recognized as Safe by the US Food and Drug Administration and other regulatory agencies. Terpenoids are quite potent, and affect animal and even human behaviour when inhaled from ambient air at serum levels in the single digits ng·mL−1. They display unique therapeutic effects that may contribute meaningfully to the entourage effects of cannabis-based medicinal extracts. Particular focus will be placed on phytocannabinoid-terpenoid interactions that could produce synergy with respect to treatment of pain, inflammation, depression, anxiety, addiction, epilepsy, cancer, fungal and bacterial infections (including methicillin-resistant Staphylococcus aureus). Scientific evidence is presented for non-cannabinoid plant components as putative antidotes to intoxicating effects of THC that could increase its therapeutic index. Methods for investigating entourage effects in future experiments will be proposed. Phytocannabinoid-terpenoid synergy, if proven, increases the likelihood that an extensive pipeline of new therapeutic products is possible from this venerable plant.
LINKED ARTICLES This article is part of a themed issue on Cannabinoids in Biology and Medicine. To view the other articles in this issue visit http://dx.doi.org/10.1111/bph.2011.163.issue-7
romanoweed
Well-known member
my feeling says this: "why think they have a major role" ?Something I've wondered is if terpenes are mainly responsible for the high
There are two things to say: there are 100s of Cannabinoids not studied. terpenes are a bit more studied i think for perfumery and aromatherapy i guess.. So its easy to just forget about all those Cannabioids and think they are tere for no reason. (well im not saying someone does forget em, but its just a high secultive Statement to give a Rating on the percentage of "Roleplaying" if we dont know about 100s of Cannabinoids.
I personally know about the power of Terpenes, because i make perfumes. I knwo that terpenes in the right mixture or a Single Material in good quaity can have enormous Effects. But wait, effects? So: no i dont feel high from them. I dont feel anything from perceptional Change, nor do i feel Seretonin-fluding.
I dont get that insane amount of anandamide or however its called.. (scientists say, that drugs give often the same Luckhormones as sport or sugar, but do it in much higher amounts, //unnatural amounts).
So, what do i then get?
I get somehgin that i call Emotions/Moods. I think thats my Lymbic System (the Lymbic System tels you either "stay here its good here" or "flee, its not good here". It signals you either savety, or danger, and does this of corse in all its nuances, down to telling you very distinctly what the fruit infront your nose will do for you and what it wont . (how preciese it is was a specualtion on my part, but i just wanted to say, its of corse not just : run VS stay. ..).
So, and if a perfume or single Material smell is super good , then its like your smelling ultra rich nurtients contained in rich Valleys full of Plants, something like that.. and you just mostly FEEL the MOOD of extreme wellbeing (that is if you really managed to find a good perfume wich is rare in stores).
The Percentage of roleplaying between the Cannbinoids and Terpenes, i cant possibly tell you.
But i can tell you this, even if its a small percentage : those details DO MATTER. its the same thing in Sound..
PEople tune their Guitars till oblivon, they will only change the last 5 to 10 percent of sound with it, but they do it a lifelong at times.. This is unlogic, right? One would think things need only to be mostly right.. but i say, no! we humans arent here to roundabout do things, we are here to push the limits on certain Arts, and those few percent can well be recieved, also by newbies (if they are interested in it).
So, im pretty shure there is no way around good terpenes.. What i would say , that this doesent mean one can now drop random essential oils onto buds (wich anyway is harmful) , or manipulate it. I dont want to make it appear like its a seperate thing that we can manipulate and change the Strain we are consuming.
Also: its really crazy but somehow those 5 percent DONT MATTER MUCH, as logic would imply, its a minor thing, thats why you can eat edibles with minor change , but funny in same time it is a maor Thing.. I have problems to find better words. But i stick to it, the best and secound best weed were somehow very similar, and somehow totally different.. its crazy, its like when we are smoking something close to perfection we have rpoblems even to describe the importance-factor of it.
(in cobcured the terpenes change, BUT your brain mapps things, and therefore understands that its the same thing, just one time decarbed one time not.. so they have a similar meaning to your brain.. )
romanoweed
Well-known member
im worried that AI doesent know about many true Mexican / Thai samles, and therefor dictates us what Budtenders would advise as 100 percent Sativa, lolSo i found a strain in that thread with a profile known for being visual and euphoric and it matches my description of a trippy strain:
Verdant Whisperer
Well-known member
This was from the other Article, had nothing to do with AI. but it seems to know the difference when i was talking with it between NLD and BLD and that sativa and indica are more terms used ,more to describe the effects. and original thought about this is from human brain not ai.im worried that AI doesent know about many true Mexican / Thai samles, and therefor dictates us what Budtenders would advise as 100 percent Sativa, lol
Last edited:
Verdant Whisperer
Well-known member
(A)-Those post you pointed out are not relevant to this conversation specifically, it has the word terpene in them, but the information doesn't apply directly, its good info don't get me wrong just not specific to this context. if anything the knowledge from these studies was used in finding my results.What I'm reading from your post here is that (A)terpenes have a relational effect to the cannabinoid effect for the smoker, right? This is something that has been studied for quite a while and is pretty much the prevailing thought in the community that studies these types of things. There are indeed many, many articles related to your train of thought in the link I posted. A quick ctrl+f using the word terpene brings up the below quoted article, touching on what you first posted in this thread. I wasn't trying to criticize, but point out that there are many other real people in the world who have the same questions and tested their own theories. I think it is good to learn what is out there to help in forming your own theories. (B) Basing something off results from a search engine geared to learn from and sell shit to you isn't the greatest place to start. There is some of the best cannabis information available on this site alone, you just have to learn how to find it.
(B)- Only using one site as a knowledge base(while this site has a ton), and not other sources, like search engines, and Ai to search vast articles for you would be limiting your knowledge base.
Last edited:
Verdant Whisperer
Well-known member
<3 This, awesome persepective.I personally know about the power of Terpenes, because i make perfumes.


