-
ICMag with help from Landrace Warden and The Vault is running a NEW contest in November! You can check it here. Prizes are seeds & forum premium access. Come join in!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Noreason's extractions
- Thread starter noreason
- Start date
I use PTFE sheets to spray on. It was unnecessary this time, but I used it the same to make pics. Colibrì butane. 1 liter in - around 0,25 liters out.


When almost the butane is gone I added some ISO alcohol and gently stir with a teaspoon to dissolve the BHO rapidly.



When almost the butane is gone I added some ISO alcohol and gently stir with a teaspoon to dissolve the BHO rapidly.
After 48 hours winterization, waxes are out of the solution and clearly visible on the bottom of the glass as sedimentation.

Filtered with an inox mesh

Once filtered waxes can bee seen on the meshalong some other residues.

Let's start evaporation. Used the same PTFE sheet. Important to not inhale ISO vapors.

Solvent is almost gone.

Filtered with an inox mesh
Once filtered waxes can bee seen on the meshalong some other residues.
Let's start evaporation. Used the same PTFE sheet. Important to not inhale ISO vapors.
Solvent is almost gone.
After the evaporation I ended with this:

My setup for vacuum is nothing special, just a DIY kit composed by a 2cm thick PMMA table, a 1/4HP 1stage pump and some PTFE tape to seal everything.
Other than that I also have a cheap electric heating pad to keep water hot enough.


Can't go over -920 mbar. This is the max. value I get with everything sealed perfectly.

Purging starts

My setup for vacuum is nothing special, just a DIY kit composed by a 2cm thick PMMA table, a 1/4HP 1stage pump and some PTFE tape to seal everything.
Other than that I also have a cheap electric heating pad to keep water hot enough.
Can't go over -920 mbar. This is the max. value I get with everything sealed perfectly.
Purging starts
-920 millibar = -27.167" Hg
https://www.convertunits.com/from/millibar/to/inches+of+mercury
If you're not at sea level your dial gauge isn't giving you a correct reading, here's a previous comment I made on the subject,
https://www.icmag.com/ic/showpost.php?p=7654817&postcount=5
Thanks for the nice write up and pics!!
https://www.convertunits.com/from/millibar/to/inches+of+mercury
If you're not at sea level your dial gauge isn't giving you a correct reading, here's a previous comment I made on the subject,
https://www.icmag.com/ic/showpost.php?p=7654817&postcount=5
Thanks for the nice write up and pics!!
Sky please stay on this forum and don't go away bro! we all need guys like you and many others 
I got your point about the gauge. Please, let me see if I got it...if I start the system at higher altitude I will read less than now on the gauge, but in the vac. chamber I have the same -29.9''Hg ?
Obviously as you was saying on the other post, having less ambient pressure it makes no sense the pump have less power to make vacuum.
And just one more question if you don't mind. What about the ziplock bag? What's the concept behind it?

I got your point about the gauge. Please, let me see if I got it...if I start the system at higher altitude I will read less than now on the gauge, but in the vac. chamber I have the same -29.9''Hg ?
Obviously as you was saying on the other post, having less ambient pressure it makes no sense the pump have less power to make vacuum.
And just one more question if you don't mind. What about the ziplock bag? What's the concept behind it?
You got it, it's just the gauge that's off.
A previously unopened Zip-Lock style bag has almost no air in it, but there are enough molecules to force it to expand when the vacuum gets nearly as deep as a rotary vane pump will take it. The vacuum inside the bag is the same as everywhere else within the system, therefore the expansion of the bag is the equalization of the air density inside and outside of the bag.
A previously unopened Zip-Lock style bag has almost no air in it, but there are enough molecules to force it to expand when the vacuum gets nearly as deep as a rotary vane pump will take it. The vacuum inside the bag is the same as everywhere else within the system, therefore the expansion of the bag is the equalization of the air density inside and outside of the bag.
You got it, it's just the gauge that's off.

A previously unopened Zip-Lock style bag has almost no air in it, but there are enough molecules to force it to expand when the vacuum gets nearly as deep as a rotary vane pump will take it. The vacuum inside the bag is the same as everywhere else within the system, therefore the expansion of the bag is the equalization of the air density inside and outside of the bag.
Makes all the sense, assuming bags were vacuumed at -29.9
Will give it a try and see what happen.
Thanks bro

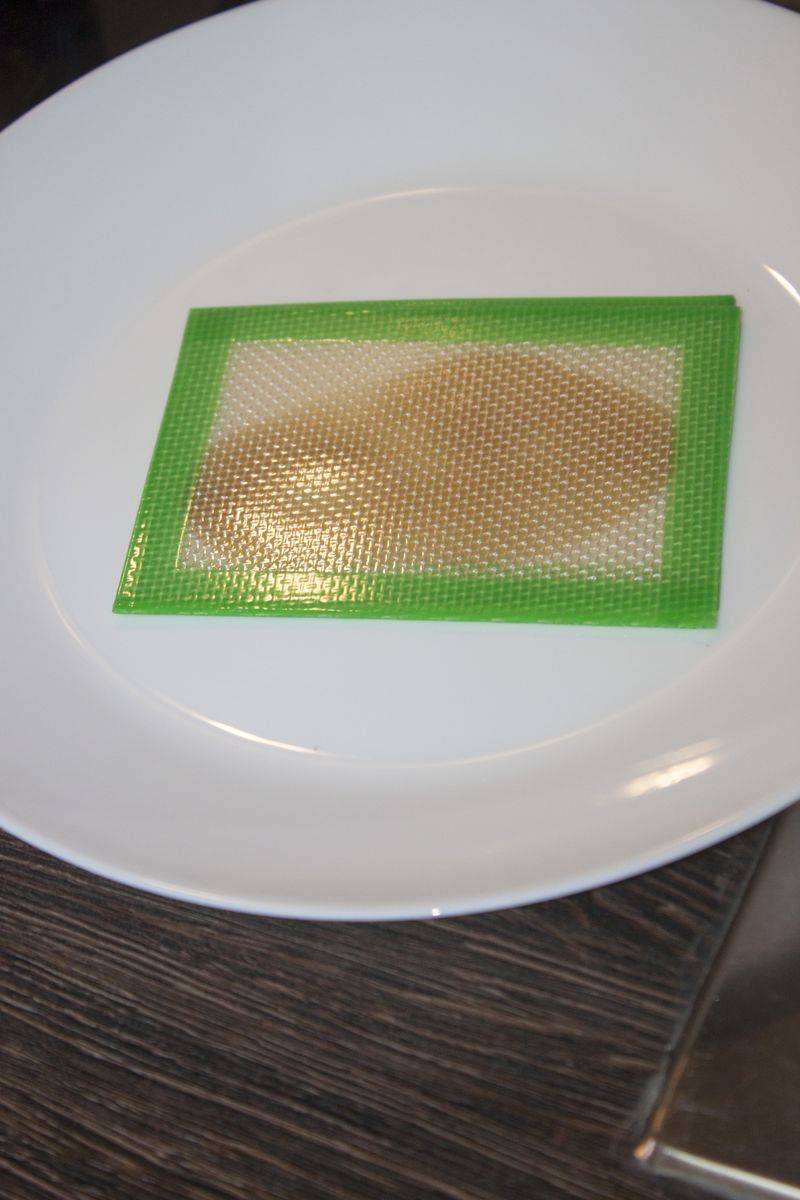
Once the first purge is done I take the extract out, fold the PTFE sheet and press it to make as thin as I can.
2 minutes in the freezer to take off the sheet from it on one side, and vacuum it again.

Same thing can be done using slickpads. To move extract from a sheet to another, or from a sheet to slickpad, just heat it up, stick the pad on the free side and then some minutes in the fridge/freezer.


Once I had it between slikpads I pressed it one more time

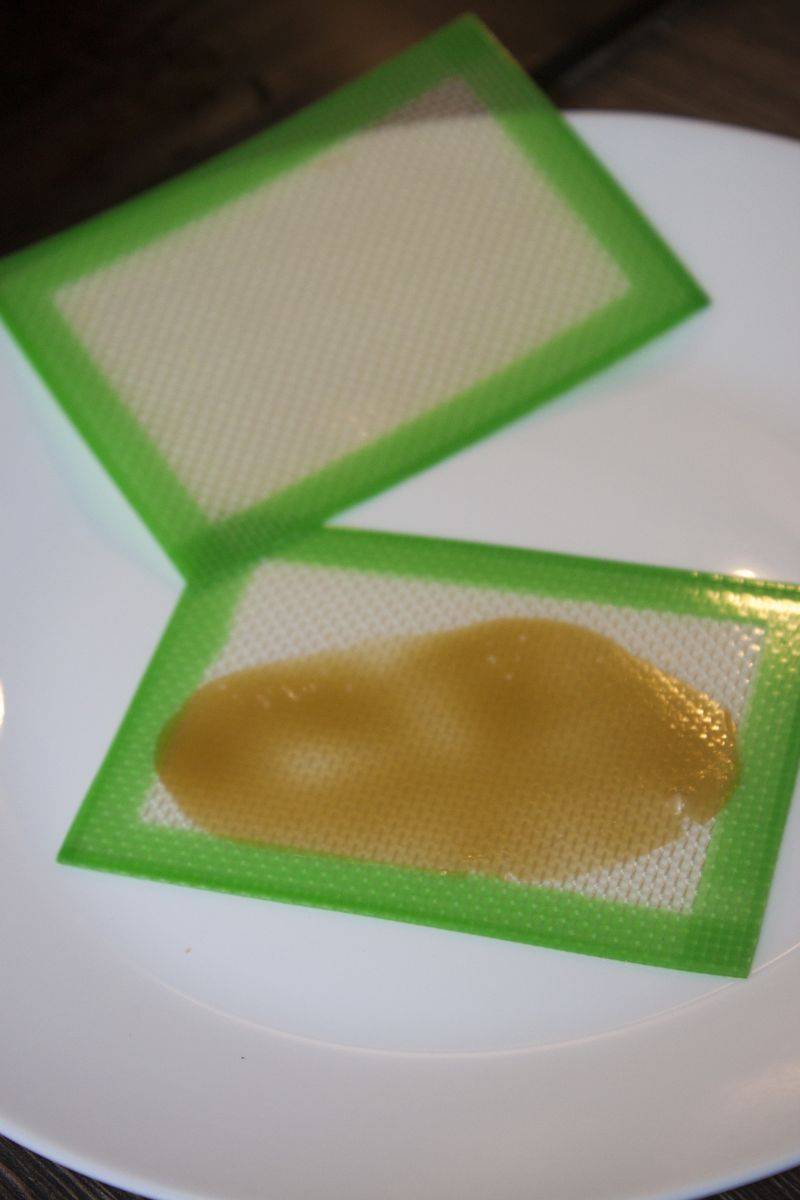
2 minutes in the freezer to take off the sheet from it on one side, and vacuum it again.
Same thing can be done using slickpads. To move extract from a sheet to another, or from a sheet to slickpad, just heat it up, stick the pad on the free side and then some minutes in the fridge/freezer.
Once I had it between slikpads I pressed it one more time
Ready for the last vacuum run

I ended with this budder
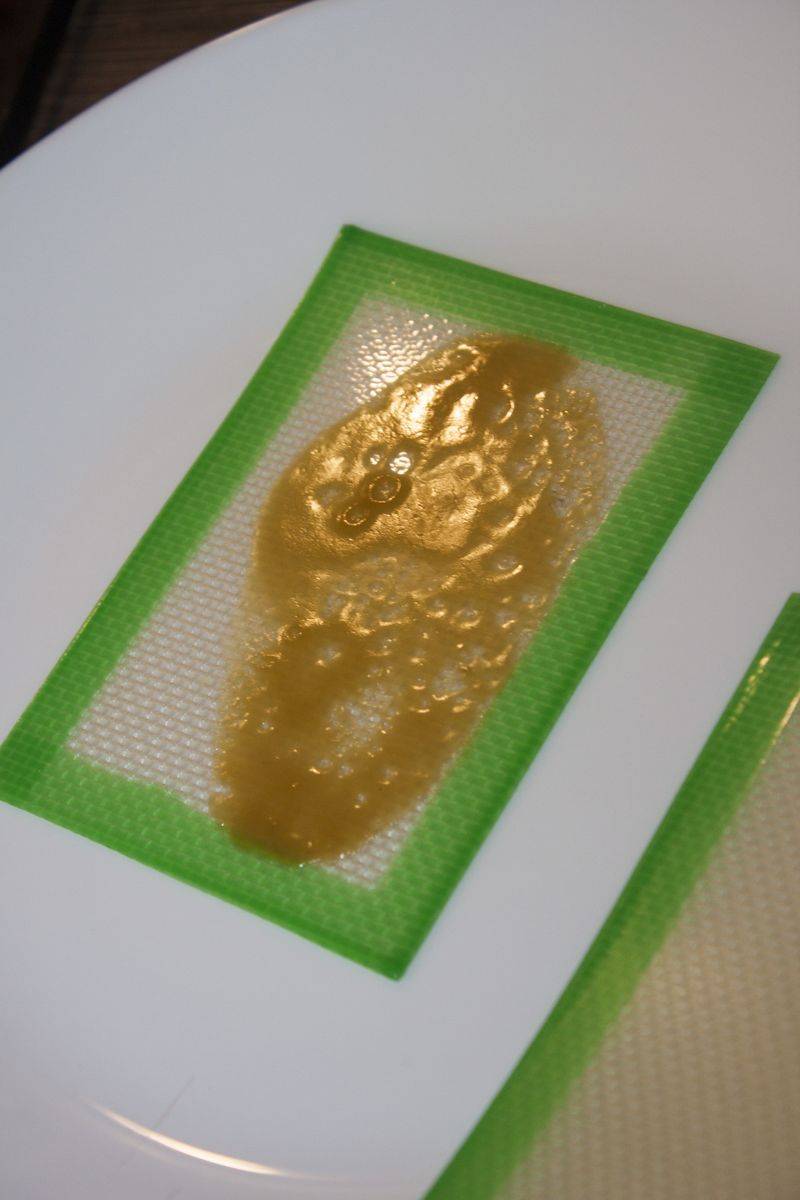

This little sample let me understand a little more. I can say it took too much heat and terps boiled off, well, not everything but a good part (the budder aspect is due to this I think).
On the other side the smoke is really smooth. Big hits on Ti-pad and no cough or any discomfort and a good high, clear one, not different from other CT extracts I made, so I guess THC and CBD are still there.
From the whole glass tube I ended with 1,37 grams of budder, what I was expecting.
Next time I'll repeat the same process but using dry ChocoTonic popcorns and small buds, staying at lower temp trying to find the right point between terps. and smoothness of smoke.
Other than that I want to split the extract and do a winterization only for an half, than vacuum both at the same time.
Green vibz!

I ended with this budder
This little sample let me understand a little more. I can say it took too much heat and terps boiled off, well, not everything but a good part (the budder aspect is due to this I think).
On the other side the smoke is really smooth. Big hits on Ti-pad and no cough or any discomfort and a good high, clear one, not different from other CT extracts I made, so I guess THC and CBD are still there.
From the whole glass tube I ended with 1,37 grams of budder, what I was expecting.
Next time I'll repeat the same process but using dry ChocoTonic popcorns and small buds, staying at lower temp trying to find the right point between terps. and smoothness of smoke.
Other than that I want to split the extract and do a winterization only for an half, than vacuum both at the same time.
Green vibz!
To all the noobs on here reading this, interested in doing such extractions....always follow the right safety rules. Butane is explosive, not just flammable, instruct yourself how to stay safe.
I always use hard equipment, gloves, mask, helmets, strong thick clothes when I work with butane. When it's evaporated than things are safe, before, better take all the preventive measures.
Other than that, one of the most difficult things for a quality extract is to keep things clean. Fuck me if I don't try to, and fuck me if only one time I ended with a really uncontaminated extract. It's near to impossible but the more you work in a dirt and dusty environment the more contaminated will be the extract

I always use hard equipment, gloves, mask, helmets, strong thick clothes when I work with butane. When it's evaporated than things are safe, before, better take all the preventive measures.
Other than that, one of the most difficult things for a quality extract is to keep things clean. Fuck me if I don't try to, and fuck me if only one time I ended with a really uncontaminated extract. It's near to impossible but the more you work in a dirt and dusty environment the more contaminated will be the extract

QuantumWeed
Member
How long do you purge it for and at what pressure?
You metioned boiling some terps, from the water temperature under the vaccum chamber?
I am experimenting myself with QWET and am worried that when boiling the Alchool I also loose the good stuff, would a vacuum chamber purge the Ethanol correctly?
I will try to use BioEthanol as it is much cheaper.
You metioned boiling some terps, from the water temperature under the vaccum chamber?
I am experimenting myself with QWET and am worried that when boiling the Alchool I also loose the good stuff, would a vacuum chamber purge the Ethanol correctly?
I will try to use BioEthanol as it is much cheaper.
How long do you purge it for and at what pressure?
Let's say some hours...never checked the time.
Lately I'm used to let it 15 minutes purging, then take it out and back in, exposing the other side of the extract to vacuum.
This process repeated various times, more than 10, works better than leaving the extract alone for hours. I can notice it by the amount of bubbles.
You metioned boiling some terps, from the water temperature under the vaccum chamber?
Yep! Because I was wrong reading the pressure and get too high with temperature.
I am experimenting myself with QWET and am worried that when boiling the Alchool I also loose the good stuff, would a vacuum chamber purge the Ethanol correctly?
Vacuum will work better and faster, however try to have a thin layer of extract and purge that. The thinner the better.
QuantumWeed
Member
Let's say some hours...never checked the time.
Lately I'm used to let it 15 minutes purging, then take it out and back in, exposing the other side of the extract to vacuum.
This process repeated various times, more than 10, works better than leaving the extract alone for hours. I can notice it by the amount of bubbles.
Yep! Because I was wrong reading the pressure and get too high with temperature.
Vacuum will work better and faster, however try to have a thin layer of extract and purge that. The thinner the better.
Below 150C should preserve all terps https://www.steephill.com/science/terpenes
Just posted my Eth extractions 'Barabba's Extractions' in this section.
Below 150C should preserve all terps https://www.steephill.com/science/terpenes
Many aromatic compounds are considered volatile because the vapor pressure (atmospherical) is not enough to keep them in that state of matter (liquid or solid) at normal temperature and pressure.
That's why you can smell weed in the air, because the aroma slowly goes in the air thanks to evaporation (it pass through the trichome's wall).
Increasing temperature and/or pressure means a faster transition. Temp. and pressure are two faces of the same coin btw

QuantumWeed
Member
Many aromatic compounds are considered volatile because the vapor pressure (atmospherical) is not enough to keep them in that state of matter (liquid or solid) at normal temperature and pressure.
That's why you can smell weed in the air, because the aroma slowly goes in the air thanks to evaporation (it pass through the trichome's wall).
Increasing temperature and/or pressure means a faster transition. Temp. and pressure are two faces of the same coin btw

So maybe solventless extractions could be better at preserving that. Thinking of getting a rosin press.




