Hydroxyl takes precedence over hydrogen.
-
As of today ICMag has his own Discord server. In this Discord server you can chat, talk with eachother, listen to music, share stories and pictures...and much more. Join now and let's grow together! Join ICMag Discord here! More details in this thread here: here.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Is the calcium in tap water available to plants?
- Thread starter beta
- Start date
Dr.Dutch
Well-known member
Yes, we were operating based on different foundational knowledge. It is clear to me that adjusting the pH of water is necessary and should be mentioned as much as the fact that plants need light to grow.I think there are some good point being made for both sides, and I think there is actually agreement. However, I believe the differences come down to the use cases and generalizations therein.
Ca and Mg is dissolved and so are the carbonates. You don't make Ca and Mg available with acid, when we talk about tap water.When you pH down carbonate laden water that releases the calcium and magnesium in free readily available form. Any time an acid (H+) comes in contact with a carbonate (-) it will reduce the atomic charge to neutral. This is an ongoing process through physical contact with the chemical compounds. It's why carbonates are called a buffer. When the carbonates compound charge is reduced to 0, all used up, then the buffering stops, all the metal elements are available until they again react with something and pH will go down or up depending on what is added pH wise
Adding acid causes a reaction with the bicarbonates and carbonates that removes them. But no effect on the calcium and magnesium.
If someone wants to know more:I won't provide the equation, as I don't understand it. I just take what I'm told with chemistry.
Why? Because hydrogen links up with something and leaves. But the alkalinity stays which takes precedence over hydrogen.Hydroxyl takes precedence over hydrogen.
Three Berries
Active member
So what happens to the Ca and Mg when the carbonates are removed? What happens to the carbonates?
if you use Nitric acid it becomes calcium nitrate and CO2
If I use my MB nute and hard well water I get a pH around 6.8, If I use the MB and rainwater I get a pH of 5.9.
Using the well water it takes about 3 to 4 hours for the bubbling to stop and reactions to stabilize the pH.
if you use Nitric acid it becomes calcium nitrate and CO2
If I use my MB nute and hard well water I get a pH around 6.8, If I use the MB and rainwater I get a pH of 5.9.
Using the well water it takes about 3 to 4 hours for the bubbling to stop and reactions to stabilize the pH.
Last edited:
Dr.Dutch
Well-known member
If we are considering a plant nutrient solution where calcium, magnesium, and carbonates are already completely dissolved, the behavior of these components can be explained as follows:
When carbonates are present in a nutrient solution, they typically exist as dissolved carbonate ions (CO₃²⁻). Calcium and magnesium ions also exist as dissolved ions (Ca²⁺ and Mg²⁺) in the solution.
If the carbonates are removed from the nutrient solution, either by chemical treatment or other means, the carbonate ions (CO₃²⁻) will no longer be present. However, the calcium and magnesium ions will remain in the solution unless they undergo further chemical reactions.
In the absence of carbonates, the calcium and magnesium ions will still be available for plant uptake as essential nutrients. They can be absorbed by the plant roots in their ionic form (Ca²⁺ and Mg²⁺) from the nutrient solution.
The removal of carbonates does not directly affect the presence or availability of calcium and magnesium ions. These ions will continue to play their roles in plant nutrition, contributing to various physiological processes within the plants.
When carbonates are present in a nutrient solution, they typically exist as dissolved carbonate ions (CO₃²⁻). Calcium and magnesium ions also exist as dissolved ions (Ca²⁺ and Mg²⁺) in the solution.
If the carbonates are removed from the nutrient solution, either by chemical treatment or other means, the carbonate ions (CO₃²⁻) will no longer be present. However, the calcium and magnesium ions will remain in the solution unless they undergo further chemical reactions.
In the absence of carbonates, the calcium and magnesium ions will still be available for plant uptake as essential nutrients. They can be absorbed by the plant roots in their ionic form (Ca²⁺ and Mg²⁺) from the nutrient solution.
The removal of carbonates does not directly affect the presence or availability of calcium and magnesium ions. These ions will continue to play their roles in plant nutrition, contributing to various physiological processes within the plants.
Looks like we'll be waiting for some time for an explanation as to why a person should think that something already dissolved in the water isn't available like it doesn't exist.
Even if you start with HNO3 added to a stirred suspension of CaCO3, you have Ca++ and NO3-.
There are some things thought of as salts, are made like salts, which dissolve in water without reacting with it but are more covalent than ionic and either do not disassociate or they latch onto something else. Mercuric chloride is a prime example. It isn't Hg++ and Cl- in water.
But that doesn't mean that's it's not bioavailable. Whether free or attached to an ionic salt it certainly is available just like your chelated micros.
if you use Nitric acid it becomes calcium nitrate
Even if you start with HNO3 added to a stirred suspension of CaCO3, you have Ca++ and NO3-.
There are some things thought of as salts, are made like salts, which dissolve in water without reacting with it but are more covalent than ionic and either do not disassociate or they latch onto something else. Mercuric chloride is a prime example. It isn't Hg++ and Cl- in water.
But that doesn't mean that's it's not bioavailable. Whether free or attached to an ionic salt it certainly is available just like your chelated micros.
Three Berries
Active member
That's how they make Calcium Nitrate
Last edited:
That's how they make Calcium Nitrate
To joes point, when an ionic species ( what we call “salts” are dissolved in water they dissociate into their respective ions. So when calcium nitrate is dissolved, you end up with transient calcium 2+ ions coordinated by water and other ligands, and nitrate anions floating around. To get there pure species, you need to remove all solvent (Water). it sounds kind of semantic, but it’s important for chemistry.
Back to the carbonate/bicarb discussion. I still maintain that the counter ion is important. For example, once your soluble ion reaches the root hair, it still needs to be absorbed. Major pathways I’m aware of are ion channels and transport proteins. H+ dependent pathways rely on a proton gradient to selectively grabs ions like calcium. That is to say they exchange H+ for Ca2+ to balance charge. If they dump a proton off, and the pH is high, that proton will be lost as CO2 and water. If however, the counter ion is something else, say nitrate, that proton is not lost and the intended reaction can take place.
Hiddenjems
Well-known member
You can use the minerals in tap water for your plants if they’re in relatively low concentration and you know what you have.
That being said, the first time you have an issue you can’t figure out, you’re going to have to explain your Frankenstein water solution to people.
Ro puts everyone around the world on the same playing field, no different conditions at all.
That being said, the first time you have an issue you can’t figure out, you’re going to have to explain your Frankenstein water solution to people.
Ro puts everyone around the world on the same playing field, no different conditions at all.
Dr.Dutch
Well-known member
In the equation, no water exists on the left side. This has absolutely nothing to do with our case, where calcium is only a few parts per million and the predominant part is H2O.That's how they make Calcium Nitrate
If you are interested in more detail, then deal with how salts dissociate in water, how it is with the strength of the chemical bonds and how the ions react with water.
A bit simplified: H2O is a weak base and acid at the same time, which interacts with ions. That is why calcium nitrate does not form in solution: calcium is too strongly bound to water and no longer forms a bond with nitrate.
Ca++
Well-known member
This got a lot more in depth than expected.
I have a practical situation that suggests availability should be graded, not just looked at as yes or no.
My tap is 350 of hardness, 280 of which is calcium carbonate. I live on sand stone.
That is a lot of Ca yet I still add some sometimes. Just another 20ppm from the bottle, makes a difference that takes me from lacking to over abundant. Unfortunately I can't say what the Ca source is, but it's from the Mono range, and lists nothing else.
So are all Ca forms equal. Or is the carbonate in question really in need of the H ions, best accomplished in waters where acid can be used.
I have a practical situation that suggests availability should be graded, not just looked at as yes or no.
My tap is 350 of hardness, 280 of which is calcium carbonate. I live on sand stone.
That is a lot of Ca yet I still add some sometimes. Just another 20ppm from the bottle, makes a difference that takes me from lacking to over abundant. Unfortunately I can't say what the Ca source is, but it's from the Mono range, and lists nothing else.
So are all Ca forms equal. Or is the carbonate in question really in need of the H ions, best accomplished in waters where acid can be used.
Dr.Dutch
Well-known member
No, let me clarify again: When it is dissolved, it doesn't matter. In the Tab water, a portion can indeed exist as undissociated calcium bicarbonate, if I can trust GPT on that.This got a lot more in depth than expected.
I have a practical situation that suggests availability should be graded, not just looked at as yes or no.
My tap is 350 of hardness, 280 of which is calcium carbonate. I live on sand stone.
That is a lot of Ca yet I still add some sometimes. Just another 20ppm from the bottle, makes a difference that takes me from lacking to over abundant. Unfortunately I can't say what the Ca source is, but it's from the Mono range, and lists nothing else.
So are all Ca forms equal. Or is the carbonate in question really in need of the H ions, best accomplished in waters where acid can be used.
If we look at the dissociation of carbonate, we see that we are discussing only bicarbonate and not carbonate.
Wait a moment, this I have noticed that before. Let's ask GPT:
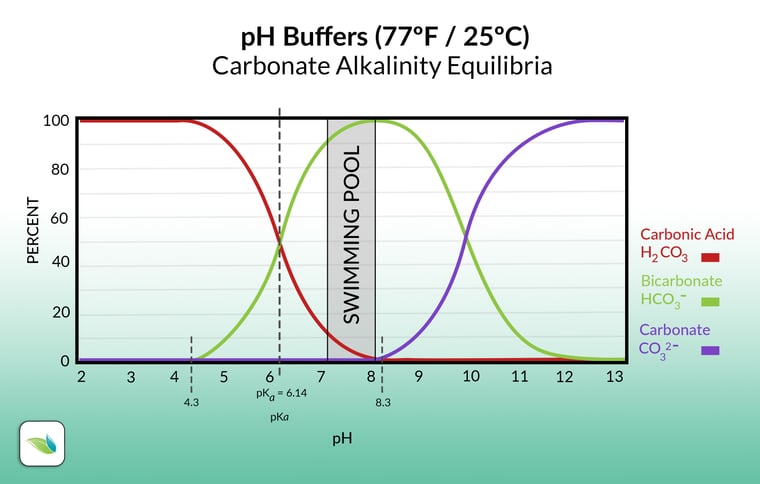
Okay, this looks good. Fits the graphOk, another question: What happens exactly with the bicarbonat in a solution (as discused) when we add a acid? It looks like hydrogen is only present between a pH of 1 and 4 and converts to carbonic acid at lower values
When an acid is added to a solution containing bicarbonate ions (HCO3-), several reactions can occur depending on the pH and the strength of the acid. Let's explore the reactions that can take place:
In summary, when an acid is added to a solution containing bicarbonate ions (HCO3-), the bicarbonate ions can act as both a weak base and a weak acid, depending on the pH conditions. The reaction can lead to the formation of carbonic acid (H2CO3), which can further dissociate into water (H2O) and carbon dioxide (CO2) at very low pH values. The exact behavior of the bicarbonate ions depends on the specific acid used, the pH of the solution, and the concentration of the bicarbonate ions.
- At higher pH (above 8.3): Bicarbonate ions (HCO3-) predominantly exist in the solution. They can act as a weak base and accept a proton (H+) from the acid, forming carbonic acid (H2CO3).
- At moderate pH (between 4 and 8.3): The bicarbonate ions (HCO3-) remain stable in the solution, as they are not significantly affected by the acid. Carbonic acid (H2CO3) is present in small amounts.
- At lower pH (below 4): As the pH decreases, more protons (H+) are available in the solution. The bicarbonate ions (HCO3-) can act as a weak acid, releasing a proton (H+) and converting into carbonic acid (H2CO3).
- At very low pH (around 1-2): Carbonic acid (H2CO3) can further dissociate into water (H2O) and carbon dioxide (CO2) due to the increased concentration of protons (H+) from the strong acid. This reaction is favored at extremely low pH values.
However, your calcium remains completely intact. At a pH of around 6, there will also be very little bicarbonate present, as it continues to convert into carbonic acid and escapes from the solution (carbonate-carbonic acid equilibrium).
You can make it available to plants via a function called chelation,,,it's where an amino grabs onto an element and makes it available to the plant
Three Berries
Active member
I grow in a soil medium.In the equation, no water exists on the left side. This has absolutely nothing to do with our case, where calcium is only a few parts per million and the predominant part is H2O.
If you are interested in more detail, then deal with how salts dissociate in water, how it is with the strength of the chemical bonds and how the ions react with water.
A bit simplified: H2O is a weak base and acid at the same time, which interacts with ions. That is why calcium nitrate does not form in solution: calcium is too strongly bound to water and no longer forms a bond with nitrate.
Tony Tokes
Active member
For extra calcium & a touch a sulfur I use epsom salt mix. But if you want to get essential trace minerals there’s this stuff I want to try called drops of balance .. supposed to be good stuff.
King's Indican
New member
, i liked this thread. according to a report in 2017 ( latest i could find including caco3)
i have 200 mg/l caco3 in my tap water while calcium is 38 mg/l and magnesium is 7 mg/l
ph is 7.0-7.5
ec is 0.43
im using this water in my blumat reservoir after letting it sit for 2 days.
i did 2 organic grows using biotabs line and plain water with blumats.
always got calcium and phosphorus related deficiencies around week 7-8 with autos. I noticed this could be from salt buildup.
do you think my water is okay to use with that much caco3?
I dont see salt stains around pots since im using air pots but heard people saying extreme caco3 binds up calcium and other nutrients in soil and making them unavailable.
Should i use something like drip clean?
ata clean or t.a flash clean (florakleen)
or brita filter it and add gypsum epsom?
i have 200 mg/l caco3 in my tap water while calcium is 38 mg/l and magnesium is 7 mg/l
ph is 7.0-7.5
ec is 0.43
im using this water in my blumat reservoir after letting it sit for 2 days.
i did 2 organic grows using biotabs line and plain water with blumats.
always got calcium and phosphorus related deficiencies around week 7-8 with autos. I noticed this could be from salt buildup.
do you think my water is okay to use with that much caco3?
I dont see salt stains around pots since im using air pots but heard people saying extreme caco3 binds up calcium and other nutrients in soil and making them unavailable.
Should i use something like drip clean?
ata clean or t.a flash clean (florakleen)
or brita filter it and add gypsum epsom?
mudballs
Well-known member
If you're in soil, that amount in tap water won't do anything negative, something else is going on. Perhaps media EC is way off or overfeeding, cuz soil don't need nute waterings until mid flower, if that. I see a LOT of growers feeding in soil and it's just a recipe for problems., i liked this thread. according to a report in 2017 ( latest i could find including caco3)
i have 200 mg/l caco3 in my tap water while calcium is 38 mg/l and magnesium is 7 mg/l
ph is 7.0-7.5
ec is 0.43
im using this water in my blumat reservoir after letting it sit for 2 days.
i did 2 organic grows using biotabs line and plain water with blumats.
always got calcium and phosphorus related deficiencies around week 7-8 with autos. I noticed this could be from salt buildup.
do you think my water is okay to use with that much caco3?
I dont see salt stains around pots since im using air pots but heard people saying extreme caco3 binds up calcium and other nutrients in soil and making them unavailable.
Should i use something like drip clean?
ata clean or t.a flash clean (florakleen)
or brita filter it and add gypsum epsom?
King's Indican
New member
5g pots lightmix and worm humus. im feeding plain water most of the amendments are dry.
mudballs
Well-known member
Maybe the soil composition needs looking at, or your dressing timings...that tap water is fine.5g pots lightmix and worm humus. im feeding plain water most of the amendments are dry.