Dr.Dutch
Well-known member
Using air pumps and bubblers to oxygenate a reservoir for the roots/plants is completely unnecessary and bro-science when growing in coco substrate. Coco coir naturally contains a lot of air, providing ample oxygen to the roots. This practice is only relevant in water-based systems like Deep Water Culture (DWC), where maintaining dissolved oxygen levels in the water is crucial.
Water circulation in your reservoir for coco is only necessary to prevent the water from becoming anaerobic.
PS:
And please leave the H2O2 out of your nutrient solution. At that concentration, it does absolutely nothing against fungi or bacteria. Instead, it reacts with the chelates, potentially causing some micronutrients to precipitate out, and it disappears from the nutrient solution in a very short time. See also
Water circulation in your reservoir for coco is only necessary to prevent the water from becoming anaerobic.
PS:
And please leave the H2O2 out of your nutrient solution. At that concentration, it does absolutely nothing against fungi or bacteria. Instead, it reacts with the chelates, potentially causing some micronutrients to precipitate out, and it disappears from the nutrient solution in a very short time. See also
From these choices, both hypochlorite and hydrogen peroxide have poor disinfection performance at the concentrations tolerated by plants and are hard to maintain at the desired concentrations through an entire crop cycle without ill effects
Disinfection of nutrient solutions in recirculating hydroponic systems – Science in Hydroponics
scienceinhydroponics.com
Healthy roots minimally leach nutrients into solution [101]. In our experience, increased turbidity usually indicates unhealthy roots with carbohydrate leakage. We have found that the solution in well-aerated DWC remains clear throughout the crop cycle (months) indicating low microbial activity in the bulk solution.
Several water treatment technologies have been used to reduce disease. These include chlorination, hydrogen peroxide, filtration, and ozonation [102,103,104,105]. Some sanitizers can degrade chelates in solution [106]. Ultraviolet light has been used in recirculating systems to reduce microbial activity in solution and to help prevent disease [107], but UV photons break down chelates [108], and the chelates must be re-added. Acidic root zone conditions have also been shown to reduce disease persistence [109,110].
We have not found any of the above treatments necessary. Root-zone disease has been minimal in our systems, perhaps because the root surfaces are uniformly well aerated and the steady-state nutrient levels that come from the daily refill solution result in healthier roots.
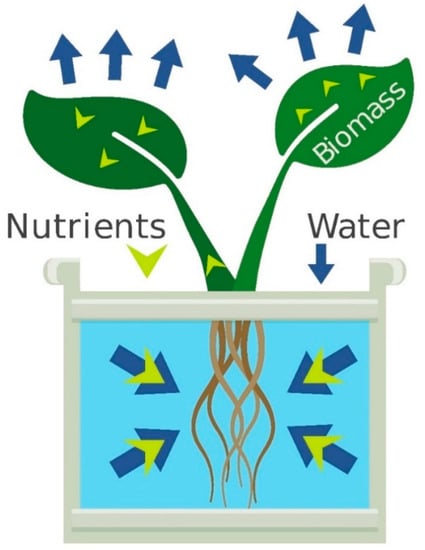
Principles of Nutrient and Water Management for Indoor Agriculture
Mass balance principles are a cornerstone of efficient fertilizer use and can be utilized to optimize plant nutrition without discarding or leaching solution. Here, we describe the maintenance of closed hydroponic and soilless substrate systems based on mass balance. Water removed by...www.mdpi.com
Last edited: