[Maschinenhaus]
Active member
Chapter 8
Indoor cannabis cultivation - Many problems?
Many problems can be prevented in advance, good gardeners know it, less is more!
Interactions between nutrients - causes of malnutrition. Causes of nutrient deficiencies are inharmonious nutrient ratios in the soil, in addition to insufficient supply due to soil depletion, nutrient leaching, pH-related establishment and oxygen deficiency.
Thus, a mutual influence of plant nutrients can occur due to uncontrolled fertilization measures, i.e. nutrients promote each other (synergism) or inhibit each other (antagonism) in the uptake.
Good, properly matured and stored compost actually contains everything we need to cultivate cannabis. We can forget about the marketing from the cannabis industry.
Useless products like bottled phosphorus, we can forget, bottled Ca-Mg, we can forget, the list would be long.
I water my plants with pure water from a reverse osmosis system, what the plant needs, it finds in the soil. I make sure that the soil is permeable to air and not too compressed. The soil must be able to breathe, water that seeps slowly brings oxygen and carbon dioxide into the soil.
My recycled Soil, after a year of rest and maturation in the cellar.

As photosynthesis increases, the plant utilizes more nutrients, growth increases with supply. m the range of optimum temperature in conjunction with high illumination, the amount of nutrients should be increased;
low soil temperature lowers nutrient uptake → supply must be reduced!
High salt content increases the salt concentration in soil and substrate, in extreme cases salt damage can occur;
special attention must be paid to sodium and chloride content;
existing nutrients may have to be counted towards fertilization; soft water (< 8° dH carbonate hardness) lowers the pH, hard water increases it, possible consequences are determination or undesired release of nutrients.
The nutrients whose study is of greatest importance are nitrogen (N) and potassium (K). They are taken up by the plant in the greatest quantity and their optimum range in the plant (especially nitrogen) is relatively narrow, so that deficiency or excess nutrition is visibly apparent even with small deviations from the optimum.
Nitrogen nutrition is influenced by the humus content of the soil or substrate in addition to fertilization. Humic mineral soils and substrates supplied with organic fertilizers or containing compost are biologically highly active. Mineralization of organic matter initially releases plant-available mineral nitrogen as ammonium, which is converted to nitrate within a few days. Prerequisites are a slightly acidic pH and normal oxygen supply.
The conversion rate increases with increasing heat supply, so that excessive levels can occur in the summer months. Nitrogen fertilization should then be interrupted. In non-active or low-active substrates (white peat / clay mixture), these difficulties do not occur to the same extent.
In contrast, the effect of phosphate is less critical, since the optimum range is very wide and excess symptoms do not occur in practice. Often greenhouse soils are oversupplied with phosphate, so that fertilization can be dispensed with even for several years. The high contents are based on the behavior of phosphate in the soil. Depending on the pH value, insoluble salts are formed over a longer period of time, namely iron and aluminum phosphate in the acidic range, calcium phosphate in the weakly acidic to alkaline range. All compounds accumulate in the soil and are not washed out. Besides iron, other trace nutrients such as zinc, boron, copper and manganese are affected by the fixation in the acidic range.
Nutritional disorders frequently detected by soil analysis concern the potassium/magnesium ratio. A balanced equilibrium exists in mineral soils at a ratio of 2 to 3 : 1. Because of the intensive rooting of pots, nutrient ratios are less important, but observance prevents serious fertilization errors. Higher magnesium contents inhibit potassium uptake, high potassium contents inhibit magnesium uptake.
It should also be noted that a wide nitrogen/potassium ratio caused by excess nitrogen will result in relative potassium deficiency. Although the potassium content in the plant is at its optimum, it falls into relative deficiency due to excessive nitrogen fertilization. If, according to a soil analysis, the nutrient ratios are balanced, they will only remain harmonious if fertilizer is applied according to the nitrogen/potassium ratio specific to the plant.
Calcium and magnesium deficits and blockages
The discussions and confusion in grower circles speak for themselves on this topic.
In good soil mixtures with compost there can be no shortage!
There are blockages, imbalance of nutrient ratios, pH value, etc., I have repeatedly pointed out in previous chapters.
Leave the bottles of miracle products on the shelves, who bought a good mixture of primary rock flour, has no problems for years if he recycles this soil again and again.
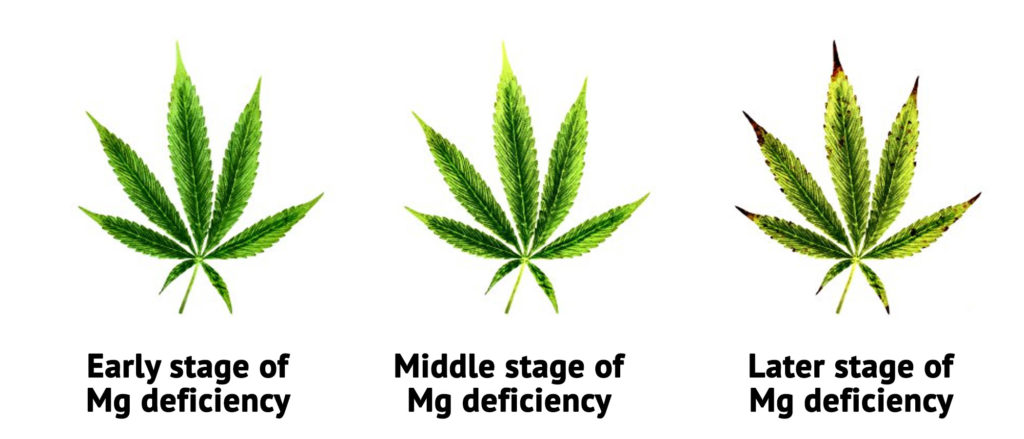
In purchased soil, even special cannabis soil, a handful of primary rock flour is enough to exclude deficiency. It is anyway the lack of magnesium that is often the cause besides blocking (see above).
Once again as a repeat!
Depending on the condition of the soil, the trace elements are susceptible to precipitation. This means that they can no longer be absorbed by the roots. In fertilizers they are therefore often processed as chelates (organo-metal complex). This protects them from blockage in the soil.
The balance between anions and cations
Under normal conditions, plants prefer to take up cations, i.e. positively charged ions, rather than the anions nitrate, phosphorus and sulfur, which are more important for nutrition.
As a result, the plant's internal charge ratios become imbalanced. To compensate, it releases hydrogen H + through the roots to the soil or substrate. This lowers the local pH value in the soil. In addition, the cations potassium K + , magnesium Mg ++ , calcium Ca ++ and ammonium NH 4 + bound to clay particles dissolve and are thus available again for the plants.
Reference
Hortipendium (German Language)
Hauert (German Language)
Indoor cannabis cultivation - Many problems?
Many problems can be prevented in advance, good gardeners know it, less is more!
Interactions between nutrients - causes of malnutrition. Causes of nutrient deficiencies are inharmonious nutrient ratios in the soil, in addition to insufficient supply due to soil depletion, nutrient leaching, pH-related establishment and oxygen deficiency.
Thus, a mutual influence of plant nutrients can occur due to uncontrolled fertilization measures, i.e. nutrients promote each other (synergism) or inhibit each other (antagonism) in the uptake.
Good, properly matured and stored compost actually contains everything we need to cultivate cannabis. We can forget about the marketing from the cannabis industry.
Useless products like bottled phosphorus, we can forget, bottled Ca-Mg, we can forget, the list would be long.
I water my plants with pure water from a reverse osmosis system, what the plant needs, it finds in the soil. I make sure that the soil is permeable to air and not too compressed. The soil must be able to breathe, water that seeps slowly brings oxygen and carbon dioxide into the soil.
My recycled Soil, after a year of rest and maturation in the cellar.
As photosynthesis increases, the plant utilizes more nutrients, growth increases with supply. m the range of optimum temperature in conjunction with high illumination, the amount of nutrients should be increased;
low soil temperature lowers nutrient uptake → supply must be reduced!
High salt content increases the salt concentration in soil and substrate, in extreme cases salt damage can occur;
special attention must be paid to sodium and chloride content;
existing nutrients may have to be counted towards fertilization; soft water (< 8° dH carbonate hardness) lowers the pH, hard water increases it, possible consequences are determination or undesired release of nutrients.
The nutrients whose study is of greatest importance are nitrogen (N) and potassium (K). They are taken up by the plant in the greatest quantity and their optimum range in the plant (especially nitrogen) is relatively narrow, so that deficiency or excess nutrition is visibly apparent even with small deviations from the optimum.
Nitrogen nutrition is influenced by the humus content of the soil or substrate in addition to fertilization. Humic mineral soils and substrates supplied with organic fertilizers or containing compost are biologically highly active. Mineralization of organic matter initially releases plant-available mineral nitrogen as ammonium, which is converted to nitrate within a few days. Prerequisites are a slightly acidic pH and normal oxygen supply.
The conversion rate increases with increasing heat supply, so that excessive levels can occur in the summer months. Nitrogen fertilization should then be interrupted. In non-active or low-active substrates (white peat / clay mixture), these difficulties do not occur to the same extent.
In contrast, the effect of phosphate is less critical, since the optimum range is very wide and excess symptoms do not occur in practice. Often greenhouse soils are oversupplied with phosphate, so that fertilization can be dispensed with even for several years. The high contents are based on the behavior of phosphate in the soil. Depending on the pH value, insoluble salts are formed over a longer period of time, namely iron and aluminum phosphate in the acidic range, calcium phosphate in the weakly acidic to alkaline range. All compounds accumulate in the soil and are not washed out. Besides iron, other trace nutrients such as zinc, boron, copper and manganese are affected by the fixation in the acidic range.
Nutritional disorders frequently detected by soil analysis concern the potassium/magnesium ratio. A balanced equilibrium exists in mineral soils at a ratio of 2 to 3 : 1. Because of the intensive rooting of pots, nutrient ratios are less important, but observance prevents serious fertilization errors. Higher magnesium contents inhibit potassium uptake, high potassium contents inhibit magnesium uptake.
It should also be noted that a wide nitrogen/potassium ratio caused by excess nitrogen will result in relative potassium deficiency. Although the potassium content in the plant is at its optimum, it falls into relative deficiency due to excessive nitrogen fertilization. If, according to a soil analysis, the nutrient ratios are balanced, they will only remain harmonious if fertilizer is applied according to the nitrogen/potassium ratio specific to the plant.
Calcium and magnesium deficits and blockages
The discussions and confusion in grower circles speak for themselves on this topic.
In good soil mixtures with compost there can be no shortage!
There are blockages, imbalance of nutrient ratios, pH value, etc., I have repeatedly pointed out in previous chapters.
Leave the bottles of miracle products on the shelves, who bought a good mixture of primary rock flour, has no problems for years if he recycles this soil again and again.
In purchased soil, even special cannabis soil, a handful of primary rock flour is enough to exclude deficiency. It is anyway the lack of magnesium that is often the cause besides blocking (see above).
Once again as a repeat!
Depending on the condition of the soil, the trace elements are susceptible to precipitation. This means that they can no longer be absorbed by the roots. In fertilizers they are therefore often processed as chelates (organo-metal complex). This protects them from blockage in the soil.
The balance between anions and cations
Under normal conditions, plants prefer to take up cations, i.e. positively charged ions, rather than the anions nitrate, phosphorus and sulfur, which are more important for nutrition.
As a result, the plant's internal charge ratios become imbalanced. To compensate, it releases hydrogen H + through the roots to the soil or substrate. This lowers the local pH value in the soil. In addition, the cations potassium K + , magnesium Mg ++ , calcium Ca ++ and ammonium NH 4 + bound to clay particles dissolve and are thus available again for the plants.
Reference
Hortipendium (German Language)
Hauert (German Language)
Last edited: