acespicoli
Well-known member
Cannabis cell culture, transformation, and micropropagation work since 1972–2020.
Front. Plant Sci., 02 March 2021
Sec. Crop and Product Physiology
Volume 12 - 2021 | https://doi.org/10.3389/fpls.2021.627240
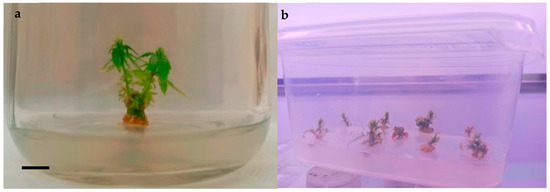
Establishment of an Efficient In Vitro Propagation Protocol for Cannabis sativa L. subsp. ruderalis Janish
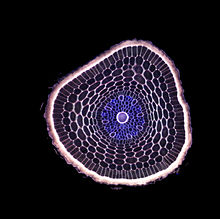
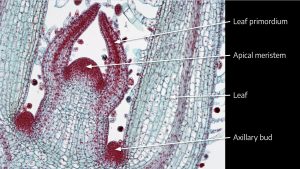
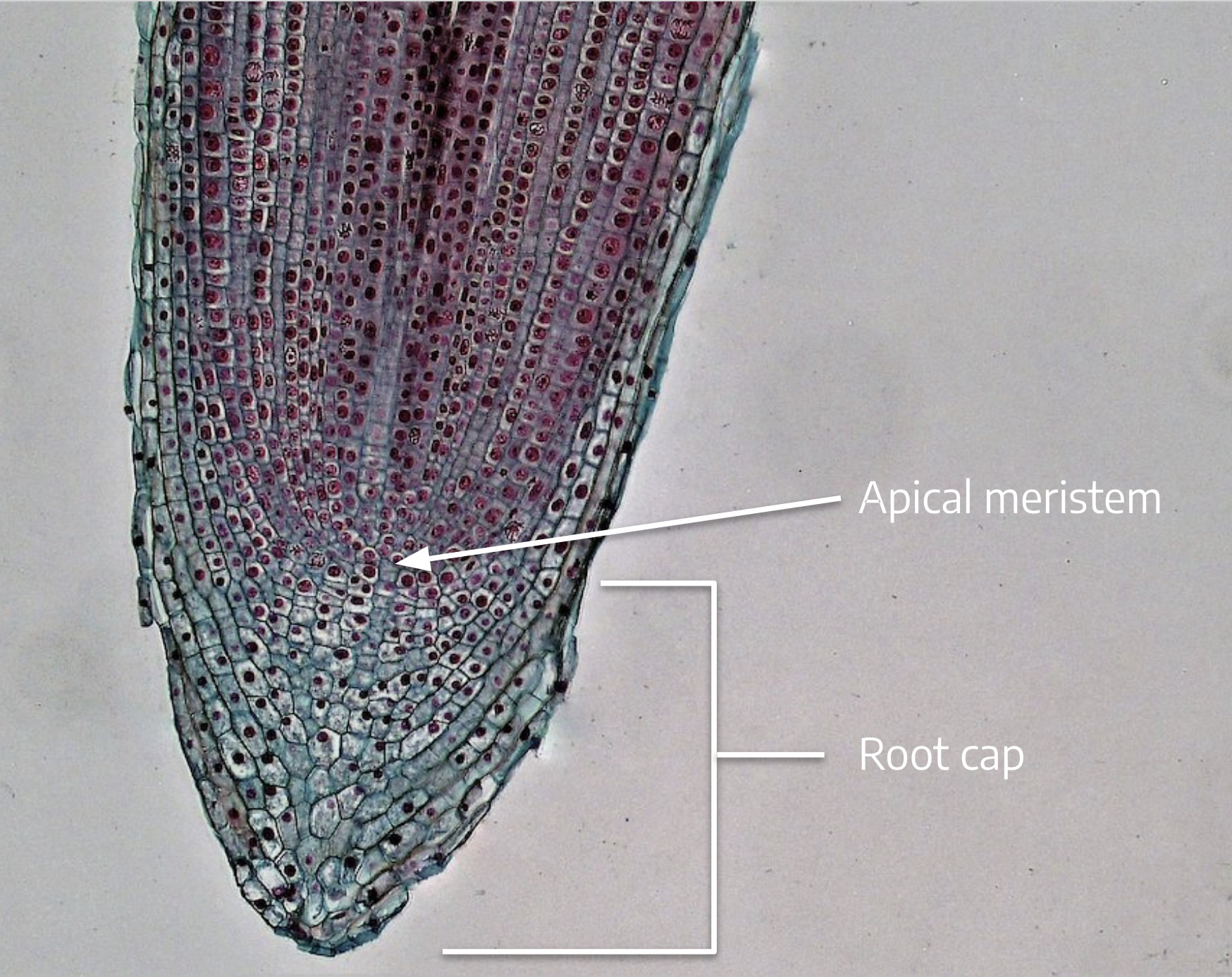
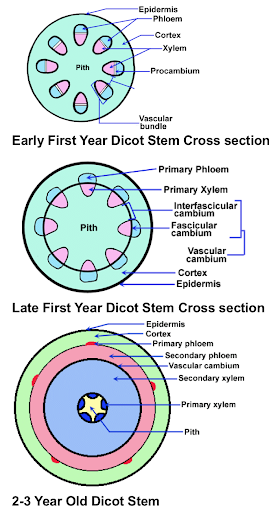
Cannabis sativa L., subsp. ruderalis Janish., ‘Finola’ is a dioecious cultivar of Finnish origin. This cultivar is very interesting because its cultivation cycle lasts less than 3 months. The aim of this study was to define an efficient micropropagation protocol to ensure in vitro multiplication...
Clonex Clone Solution is specifically formulated to provide rooted clones and seedlings with a special blend of the highest quality minerals including Nitrogen, Phosphorus, Potassium, and Calcium plus other essential elements that all plants require for vigorous growth. Clonex Clone Solution also contains Vitamin B1 which reduces the risk of transplant shock.
Used with Clonex Rooting Gel or any other rooting agent, it encourages rapid root development while helping to minimize stress. Clonex Clone Solution is the result of extensive horticultural research and is designed to work together with Clonex Rooting Gel or other rooting agents for outstanding results.
Clonex Clone Solution contains specific micro nutrients and root enhancing agents, carefully formulated to initiate and nourish new root cells in plants. Unlike competitive products, Clonex Clone Solution is highly concentrated.
***
Clonex Gel is a high performance, water-based, rooting compound. This tenacious gel which will remain in contact around the stem, sealing the cut tissue and supplying the hormones needed to promote root cell development and vitamins to protect the delicate new root tissue. Clonex has a full spectrum of mineral nutrients and trace elements to nourish the young roots during their important formative stages. The scientific breakthrough puts Clonex Rooting Gel years ahead of manufacturers of old fashioned hormones and powders.
Since 1988 Clonex Rooting Gel has led the way in plant propagation with billions of clones successfully rooted, including virtually every species of plant known to man. The Clonex formulation has been fine-tuned to give the explosive root development and for this reason serious growers and plant nurseries depend upon it for successful and profitable plant propagation. It is completely alcohol-free.
Clonex Rooting Gel is widely recognized as an industry standard for its ability to meet the successful and consistent results required for propagation done with vegetative cuttings. It is registered with the EPA and is approved for use on all food crops, including medicinal plants, in all 50 states plus Washington DC and Puerto Rico and is the only rooting gel approved by the Colorado Department of Agriculture for propagating medicinal plants.
HOW TO USE CLONEX ROOTING GEL:
First, dip cutting in gel to desired depth. Then insert cutting into rooting medium. Mist cuttings and place in propagator or a warm, clean, moist and humid environment. Finally, look for root development in 1 – 2 weeks.As a matter of fact, you can use Clonex Rooting Gel on all types of plant cuttings, including woody, herbaceous, and flowering ornamental species, vegetables, fruit trees and small fruits.
HDI-Clonex-Propagation-Guide.pdf
 drive.google.com
drive.google.com
CLONE KING
Last edited: