-
As of today ICMag has his own Discord server. In this Discord server you can chat, talk with eachother, listen to music, share stories and pictures...and much more. Join now and let's grow together! Join ICMag Discord here! More details in this thread here: here.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Cannabis Seed Storage
- Thread starter acespicoli
- Start date
acespicoli
Well-known member
Method 2
Freeze Drying with Dry Ice
-

1
Wash the produce you want to freeze dry and cook any meat. Wash fruit and vegetables under cool running water before patting each item dry with a paper towel. If you’re drying poultry, beef, or fish, make sure to cook them first.[13]- Pasta noodles should be cooked as well.
- You don’t need to wash cheeses before you freeze dry them.
-

2
Slice larger items into chunks about 1–2 inches (2.5–5.1 cm) across. Use a sharp knife to slice larger fruit and vegetables into small chunks about 1 inch (2.5 cm) to 2 inches (5.1 cm) across. If you’re freeze drying cooked meat, slice it into slivers less than 1 inch (2.5 cm) thick. Try to make each piece the same size so they freeze dry at the same rate.[14]- Small fruits like blueberries, raspberries, and blackberries can be freeze dried whole.
- Slice larger pieces of produce like potatoes, apples, and pears into smaller chunks.
- If you’re freeze drying a loaf of bread, use a serrated knife to cut it into slices about 1⁄2 inch (1.3 cm) thick.
-

3
Put the chopped food chunks into freezer bags and seal the bags. Put the sliced chunks into freezer bags. Be sure to put only 1 type of food per bag rather than mixing different kinds of foods together. Then, push out all of the air from the bags with your hands or by rolling the air out (toward the opening) with a rolling pin.[15]- Pushing out the air will ensure that no ice crystals form on the food.
-

4
Choose a storage box large enough that the bags only fill it half way. A large styrofoam cooler or large plastic container with a lid will work nicely. Note that the box will have to fit inside of your freezer, so if you have a small freezer, you may only be able to freeze dry small quantities of food at a time.[16]- Pick a plastic container you don’t plan to use for other purposes because you’ll need to put holes in the lid.
-

5
Pour 1 pound (0.45 kg) of dry ice into the bottom of the box. Put on heavy duty gloves like leather or work gloves to pour dry ice over into the bottom of the box until it forms an even layer. The amount of dry ice you need to use is equal to the weight of the food. So if you’re freezing 5 pounds (2.3 kg) of food, you’ll need about 5 pounds (2.3 kg) of dry ice.[17] If it doesn’t cover the entire bottom of the box, add another 1 pound (0.45 kg) until it does.- Depending on the width and length of the box, 5 pounds (2.3 kg) of dry ice should be enough for up to 4 layers of food.
- Don’t touch the dry ice with your bare hands—it will burn your skin! If you don’t have heavy duty or leather gloves, use oven mitts or thick kitchen towels.
- Purchase dry ice cubes online or at your local grocery store or supermarket.
-

6
Sandwich the food bags between layers of dry ice. Layer the bags on top of the bottom level of dry ice and then pour in another 1 pound (0.45 kg) to 2 pounds (0.91 kg) of dry ice to completely cover the bags. Make sure not to stack two bags directly on top of each other.[18]- You may need to rearrange the pieces of dry ice so that the bags are fully covered.
- Make sure each bag lays as flat as possible and that there’s no overlapping.
-

7
Add a final layer of dry ice on top of the food bags. Depending on the size of the box and the number of bags you have, you may need to do a few alternating layers of dry ice and food bags. Each layer of food should have dry ice on top of and underneath it.[19]
-

8
Poke a few holes into the lid and attach it to the box. Use a box cutter or sharp knife to cut 3 to 4 holes into the top of the box. These holes allow gas and moisture to escape, which is necessary for the dry ice to dissipate and for the food to fully dry.[20]- Avoid poking too many holes into the lid. The idea is to allow the gas to escape at a relatively slow rate.
-

9
Place the box into the freezer for at least 24 hours. The food is done freezer drying when all of the dry ice has disappeared. This could take 24 hours or more depending on how many layers of food you’re freeze drying (and how much dry ice you’ve used to cover it). Wear gloves to remove the lid of the box and look into the container.[21]- If you don’t see any dry ice on top, shuffle the bags around with a gloved hand to check for dry ice on the bottom. If it’s all gone, the food is ready for storage.
- If you see any chunks of dry ice, reattach the lid, reinsert the box into the freezer, and wait for 3 to 6 hours before checking again.
-

10
Store freeze-dried foods in freezer bags at room temperature. Since the foods are already in freezer bags, you can just take them out and put them in your pantry or anywhere that’s at or below room temperature.[22]- The freeze-dried food will stay good for up to 25 years.
- Eat the freeze-dried chunks as is or rehydrate them by placing them in a small amount of water.

acespicoli
Well-known member
acespicoli
Well-known member
Pollen stored
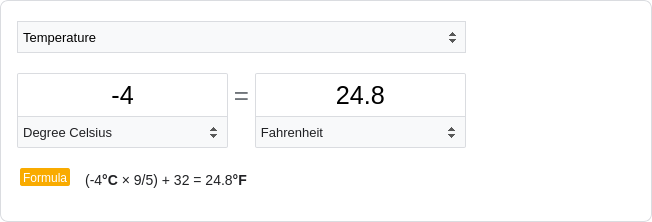
under freezer conditions (-4˚C) maintained high non-abortion rates even after 96 weeks of
storage, and the associated linear model predicted that pollen stored under freezer conditions
may maintain some intact regenerative nuclei up to 261.5 weeks, approximately five years after
anther dehiscence

 doi.org
doi.org


 en.wikipedia.org
en.wikipedia.org
under freezer conditions (-4˚C) maintained high non-abortion rates even after 96 weeks of
storage, and the associated linear model predicted that pollen stored under freezer conditions
may maintain some intact regenerative nuclei up to 261.5 weeks, approximately five years after
anther dehiscence
Methods for characterizing pollen fitness in Cannabis sativa L.
Pollen grains are male gametophytes, an ephemeral haploid generation of plants, that commonly engage in competition for a limited supply of ovules. Since variation in reproductive capabilities among male gametophytes may influence the direction and pace of evolution in populations, we must be...

Fractal - Wikipedia
Last edited:
acespicoli
Well-known member
Pollen stored under room temperature conditions (22 ± 0.95˚C) quickly declined in
viability, reaching 0% germination two weeks after anther dehiscence.
PLOS ONE | https://doi.org/10.1371/journal.pone.0270799 July 7, 2022
viability, reaching 0% germination two weeks after anther dehiscence.
PLOS ONE | https://doi.org/10.1371/journal.pone.0270799 July 7, 2022
acespicoli
Well-known member
The linear regressions used to model the relationship between storage time and abortion rates
predicted that all pollen grains would degrade at
38.3 weeks under room temperature conditions (Adj. R2 = 0.8903),
and
261.5 weeks under freezer conditions (Adj. R2 = 0.8991),
suggesting that long-term storage of pollen samples for genotyping is feasible.
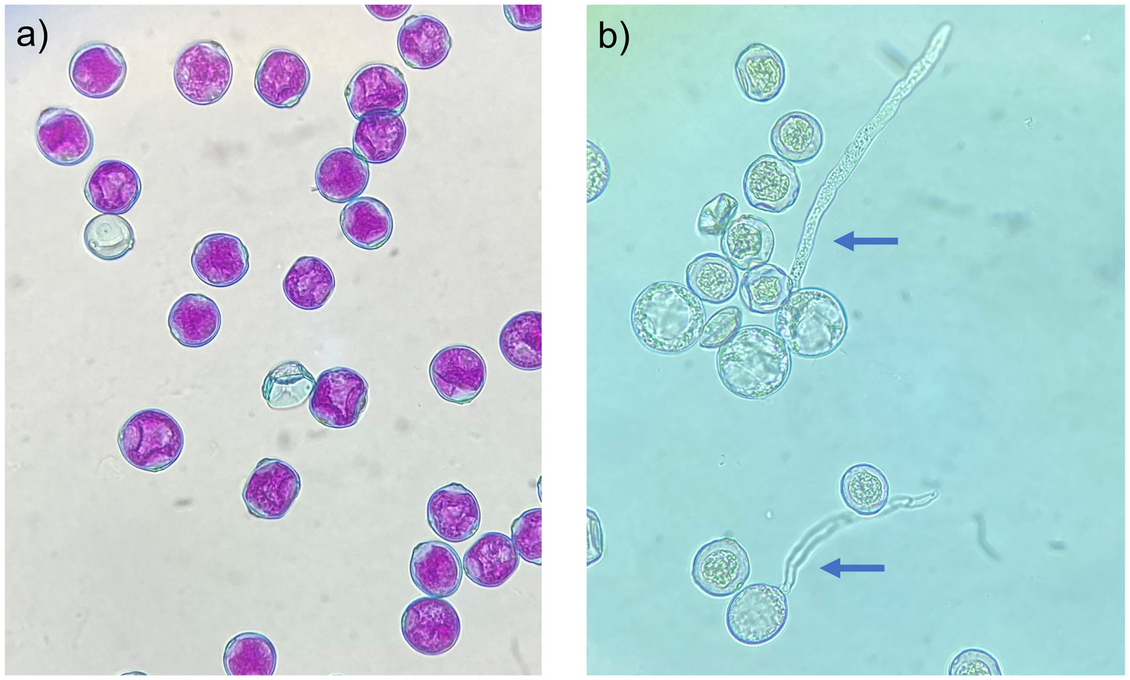
Fig 1. Differential staining and in vitro germination of Cannabis sativa pollen.
(a) Differential staining of non-aborted (pink cytoplasm with blue exines) and aborted pollen grains (blue exines), showing absorption of pink acid fuchsin in the cytoplasm of functional pollen grains. (b) In vitro germination of viable and inviable pollen grains, showing protrusion of the pollen tube in viable pollen grains (blue arrows).
PLOS ONE | https://doi.org/10.1371/journal.pone.0270799 July 7, 2022
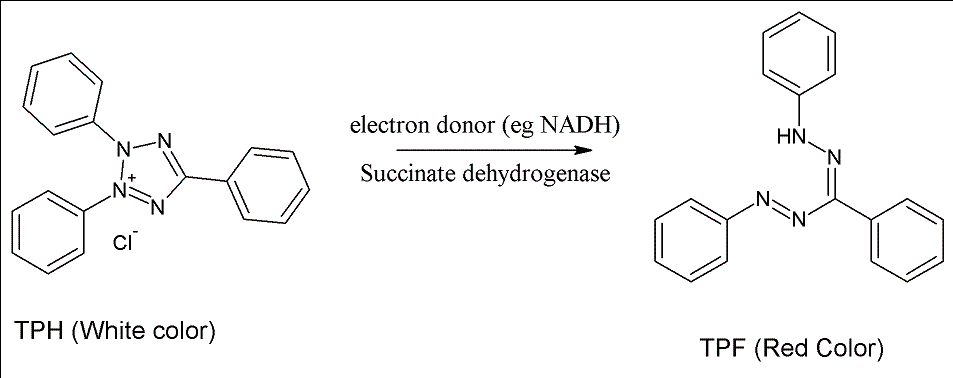
Tetrazolium Red is a colorless, water-soluble dye that is reduced to a deep red,
water-insoluble compound (formazan) mainly in the mitochondria of living cells,
predicted that all pollen grains would degrade at
38.3 weeks under room temperature conditions (Adj. R2 = 0.8903),
and
261.5 weeks under freezer conditions (Adj. R2 = 0.8991),
suggesting that long-term storage of pollen samples for genotyping is feasible.
Fig 1. Differential staining and in vitro germination of Cannabis sativa pollen.
(a) Differential staining of non-aborted (pink cytoplasm with blue exines) and aborted pollen grains (blue exines), showing absorption of pink acid fuchsin in the cytoplasm of functional pollen grains. (b) In vitro germination of viable and inviable pollen grains, showing protrusion of the pollen tube in viable pollen grains (blue arrows).
PLOS ONE | https://doi.org/10.1371/journal.pone.0270799 July 7, 2022
Tetrazolium Red is a colorless, water-soluble dye that is reduced to a deep red,
water-insoluble compound (formazan) mainly in the mitochondria of living cells,
Last edited:
acespicoli
Well-known member
acespicoli
Well-known member
simple fridge/freezer can
acespicoli
Well-known member
acespicoli
Well-known member
Dumigan, C.R.; Deyholos, M.K. Cannabis Seedlings Inherit Seed-Borne Bioactive and Anti-Fungal Endophytic Bacilli. Plants 2022, 11, 2127. https://doi.org/10.3390/plants11162127
All 15 accessions of Cannabis, including hemp and marijuana,
inherited seed-borne Paenibacillus mobilis with the capacity to solubilize mineral rock phosphate.
acespicoli
Well-known member
Abstract
As the industrial hemp (Cannabis sativa L.) market grows, there is a need for methods to clonally propagate parental breeding stock and new cultivars. Information is lacking on vegetative cutting propagation of hemp. We evaluated how propagation environment (intermittent mist vs. subirrigation under a humidity dome), indole-3-butyric acid (IBA) formulation (talc rooting powder vs. IBA in solution), and IBA concentration (0, 3000, or 8000 ppm) affected stem cuttings from ‘I3’, a cannabinoid-free cultivar of industrial hemp. Under mist or domes, rooting quality and percent declined at 8000 ppm IBA. Root and shoot quality and rooting percentage also were reduced in 3000 ppm IBA in solution treatment compared with talc. Our data show that for the cultivar tested, cuttings rooted at the highest percentage and produced the highest-quality roots and shoots with either no hormone or 3000 ppm talc powder. These treatments did equally well under humidity domes or intermittent mist.Keywords: Cannabis sativa; clonal propagation; humidity; IBA; intermittent mist; quick dip IBA; talc
The non-recreational hemp (Cannabis sativa L.) industry, particularly the cannabidiol sector, is expanding rapidly. Commercial growers and researchers are shifting to sterile triploids to avoid pollen contamination from male hemp plants grown nearby. As such, producers need reliable and efficient methods to clonally propagate industrial hemp on a large scale. Hemp is propagated either by seed (Potter, 2009), by stem cuttings (Caplan, 2018), or in vitro (Lata et al., 2017). Stem cuttings are a common method for propagation of hemp, but many factors affect the success and quality of the cuttings.
During vegetative propagation, transpiration is minimized by using methods like intermittent mist or humidity domes to maintain a high relative humidity around cuttings until they produce roots. If the relative humidity is too low, transpiration will be increased, which can cause cuttings to wilt faster and die (Owen, 2018). Humidity domes have previously been used in other experiments involving the propagation of woody plant species, such as aspen (Populus tremuloides Michx.) and balsam poplar (Populus balsamifera L.) (Wolken et al., 2010). Intermittent mist systems are the most common method of increasing humidity during cutting propagation. These systems apply short bursts of water in small droplets to the plants every few minutes throughout the duration of the light cycle. The water bursts help to keep humidity high and to minimize the rate of transpiration. Intermittent mist further reduces vapor pressure deficit by cooling the surface of the leaf via evaporation. Humidity domes (Campbell et al., 2019; Parsons et al., 2019) and intermittent mist systems (Clarke, 1981) have both been used in hemp propagation.
Indole-3-butyric acid (IBA) is often used for rooting in commercial operations (De Klerk et al., 1999) and is available in various formulations, concentrations, and application methods. IBA can be delivered to cuttings in talc or dissolved in alcohol to be used as a quick dip, whereas the potassium salt of IBA can be dissolved in water alone. Caplan (2018) found that a 0.2% (2000 ppm) IBA gel applied to hemp cuttings doubled the rooting percentage when compared with a 2000-ppm solution of willow (Salix sp.) extract. Although growers may opt to use talc or liquid formulations, it would be useful for growers to see how a single hemp breeding line reacts to each.
The purpose of our study was to evaluate the impact of environment (dome vs. intermittent mist), IBA formulation [talc vs. IBA/naphthalene acetic acid (NAA) quick dip], and IBA concentration on rooting percent and the root and shoot quality of stem cuttings from ‘I3’ hemp.
Materials and methods
Plant material
Stock plants were maintained in a greenhouse under a 24-h photoperiod with a mean canopy light intensity of 750 µmol·m−2·s−1 using 400-W high-pressure sodium lamps (Sun System, Vancouver, WA). Stock plants were potted at the beginning of Oct. 2020 as rooted cuttings in 5-gal containers. The containers were filled with a soilless potting mix (Metro-Mix; Sun Gro Horticulture, Agawam, MA) and perlite (Supreme Perlite Co., Portland, OR) (2:1 by volume)and incorporated with 67.5 g of 18N–2.6P–9.1K controlled-release fertilizer (Harrell’s, Lakeland, FL) per 2 ft3 of soilless potting mix (Metro-Mix). Plants were fertilized weekly with water-soluble 20N–8.7P–16.6K general-purpose fertilizer (Jack’s Professional; JR Peters, Allentown, PA) at 100-ppm concentration measured by a water-powered, non-electric chemical injector (Dosatron; Dosatron International, Clearwater, FL). The stock plants were 5 months old when cuttings were collected at the end of Feb. 2021. A mixture of terminal and subterminal cuttings was collected at 1400 hr. All cuttings were ≈4–5 inches in length, and each cutting had two or three fully expanded leaves. Terminal and subterminal cuttings were randomly assigned to each treatment combination. Cuttings were rooted in 10- by 20-inch plastic trays (Hydro Crunch, Walnut, CA) with drainage that contained soilless media (Sunshine Mix, Sun Gro Horticulture) and perlite (Supreme Perlite Co.) (2:1 by volume). Each drainage tray was set inside of a solid bottom 10-inch by 20-inch plastic tray (Hydro Crunch) that held water for subirrigation. The water in the bottom tray moved up through the medium by capillary action. Twenty-five cuttings were placed in each tray.Experimental design and environment
The experimental design was a randomized complete block design with a split plot arrangement of the environmental treatments. Each individual tray with 25 cuttings was counted as an experimental unit. A plastic-tented mist bench was used for the experiment. The bench was divided in half between the two environments (mist vs. no mist), and the remaining treatments were randomized within those two sub-plots. One half of the tented mist bench was equipped with mist emitters (CoolNet Pro Fogger; Netafim USA, Fresno, CA) suspended ≈30 inches above the bench surface, and the other half of the bench did not have any mist emitters; this half was used for the humidity domes. There were two IBA formulations and three different IBA concentrations applied to cuttings in both propagation methods. There were three replications for each of the 12 treatment combinations. Thirty-six different experimental units (trays) were evaluated, and a total of 900 cuttings were used. The IBA formulations were talc rooting powder (Hormex; Brooker Chemical Corp., Chatsworth, CA) and a mixture of 10,000 ppm IBA and 5000 ppm NAA in solution (Wood’s Rooting Compound; Earth Science Products Corp., Wilsonville, OR). Both IBA formulations were applied at 0, 3000, and 8000 ppm.Cuttings were collected, treated, and placed in the tented mist bench on 25 Feb. 2021. The tented mist bench was inside of a climate-controlled glass greenhouse with day/night set temperatures of 26/15 °C, with no supplemental lighting, and bottom heat at 22 °C. The trays were randomized within each section (mist vs. no mist). Trays in each group were watered at the time of propagation. From that point on, the humidity dome group was sub-irrigated with tap water as needed. The intermittent mist group was misted for 12 s once every 45 min from 0700 to 2000 hr. The humidity domes each had two vents on top. For days 1–5 after inserting cuttings into the growing medium, both vents were kept closed. On days 6–10, both vents were opened 25%. On days 11–15, both vents were opened 50%. On day 16, both vents were fully opened and remained open until the cuttings were harvested on day 28 (24 Mar. 2021).
Assessing root and shoot quality
Twenty-eight days after initiation, all cuttings were harvested, their root and shoot quality were assessed, and a rooting percentage was calculated. Root and shoot quality were assessed by rating the shoot and root system for each cutting on a scale of 0–4 (Fig. 1), with 4 being the best quality. A rating of 0 for shoots means that the shoot remained the same size as the initial cutting and did not produce any new leaves. A rating of 0 for roots means the cutting produced no roots. The mean root quality score and shoot quality score were calculated from the group of 25 cuttings within each experimental unit and subjected to analysis of variance (ANOVA) using RStudio (ver. 4.0.2; Allaire Corp., Newton, MA). ANOVA was used to compare results among the different propagation environments (humidity dome vs. intermittent mist), IBA formulations (talc powder vs. IBA in solution), and IBA concentrations. To independently assess the main effect treatments (different combinations of propagation environment, IBA formulation, and IBA concentration), Tukey’s honestly significant difference and Fisher’s least significant difference tests were performed.
Fig. 1.
Quality ratings used to evaluate (A) shoot quality and (B) root quality 4 weeks after treatment of ‘I3’ hemp cuttings. Quality was ranked on a 0–4 relative scale, with 4 representing the highest quality rating. A rating of 0 for shoots means that the shoot remained the same size as the initial cutting and did not produce any new leaves. A rating of 0 for roots means the cutting produced no roots.
Citation: HortTechnology 32, 3; 10.21273/HORTTECH05016-21
Results and discussion
Rooting success was high in both propagation environments, and there was no significant difference between them (P = 0.28); 88% of the cuttings propagated under the humidity domes rooted, and 84% of the cuttings under intermittent mist rooted (Table 1). However, there was a difference among all treatments (P = 0.094) if α was raised to 0.1. The propagation environment had no significant effect on root quality (Table 2). However, it had a significant, but modest, effect on shoot quality (Table 2). The average shoot quality rating for cuttings propagated under humidity domes was 1.85, and the average shoot quality rating for cuttings under intermittent mist was 1.62 [P < 0.01 (Fig. 2)]. Cuttings from the intermittent mist group were generally healthy, but mild amounts of chlorosis were observed on some leaves. Cuttings under the humidity domes did not show any signs of chlorosis. Other research has demonstrated that leaf chlorosis can occur under misting (Zhang and Graves, 1995).
Fig. 2.
A representation of the two-factor indole-3-butyric acid (IBA) formulation × IBA concentration interaction. Root and shoot quality are presented on a 0–4 relative scale for ‘I3’ hemp stem cuttings, with 4 being the best quality. A rating of 0 for shoots means that the shoot remained the same size as the initial cutting and did not produce any new leaves. A rating of 0 for roots means the cutting produced no roots. Ratings are separated by treatment combinations. The IBA concentration used in each treatment combination is stated below each treatment combination. “IBA in solution” represents the IBA/naphthalene acetic acid quick dip mixture. “Mist” refers to the intermittent mist propagation environment. “Dome” refers to the humidity dome propagation environment. “Talc” refers to talc based IBA powder. Shoot and root quality ratings are the two different response variables. Error bars represent standard error of the mean. Bars within each response variable (root or shoot quality) with the same letter are not different based on Tukey’s honest significant difference test (α = 0.05). Black letters refer to shoot quality; blue letters refer to root quality; 1 ppm = 1 mg·L−1.
Citation: HortTechnology 32, 3; 10.21273/HORTTECH05016-21
Impact of Indole-3-butyric Acid Concentration and Formulation and Propagation Environment on Rooting Success of ‘I3’ Hemp by Stem Cuttings
https://doi.org/10.21273/HORTTECH05016-21 1mg/liter = 1ppm
acespicoli
Well-known member
Your friend the freezer
A benevolent tool in our trade is the refrigerator and freezer. The fridge is extremely useful in extending the longevity of seed and pollen. The trick to successful freezing is to freeze deep (-10 to -40°F/-20 to -35°C) and then keep the seed undisturbed. Hard frozen objects are very fragile. The slightest shock may shatter crucial, delicate cell structures within the seed. Double wrap the seed in paper; little manilla envelopes work great.
I like to do small amounts, in one-time-use packets, to keep waste to a minimum. Then place the wrap into a plastic freezer bag, then place the freezer bag into a plastic tub or tupperware container. Now the seed is ready for the deep-freeze. In the fridge, storing seed in airtight, brown glass jars with a little rice or other non-toxic desiccant seems to work best.
I have had pollen last for years in a deep freeze. It must be frozen immediately after fresh collection from the plant, in as low a humidity as possible (preferably 0%). I like to shake the productive male flowers over a flat and clean piece of glass. The pollen pile is sifted to rid the unwanted plant material from the pure powder.
It is also useful to cut pollen with flour to stretch the amount. A pollen-to-flour ratio of 1:10 or even 1:100 works best. The cut pollen may then be separated into small, one-time-use amounts, stored in a flap of paper and frozen the same way as the seed. The frozen pollen must be applied to the live female flower immediately after thawing to increase viability.
(excerpt from DJ Short)
A benevolent tool in our trade is the refrigerator and freezer. The fridge is extremely useful in extending the longevity of seed and pollen. The trick to successful freezing is to freeze deep (-10 to -40°F/-20 to -35°C) and then keep the seed undisturbed. Hard frozen objects are very fragile. The slightest shock may shatter crucial, delicate cell structures within the seed. Double wrap the seed in paper; little manilla envelopes work great.
I like to do small amounts, in one-time-use packets, to keep waste to a minimum. Then place the wrap into a plastic freezer bag, then place the freezer bag into a plastic tub or tupperware container. Now the seed is ready for the deep-freeze. In the fridge, storing seed in airtight, brown glass jars with a little rice or other non-toxic desiccant seems to work best.
I have had pollen last for years in a deep freeze. It must be frozen immediately after fresh collection from the plant, in as low a humidity as possible (preferably 0%). I like to shake the productive male flowers over a flat and clean piece of glass. The pollen pile is sifted to rid the unwanted plant material from the pure powder.
It is also useful to cut pollen with flour to stretch the amount. A pollen-to-flour ratio of 1:10 or even 1:100 works best. The cut pollen may then be separated into small, one-time-use amounts, stored in a flap of paper and frozen the same way as the seed. The frozen pollen must be applied to the live female flower immediately after thawing to increase viability.
(excerpt from DJ Short)
acespicoli
Well-known member
Male isolation pollen chamber
acespicoli
Well-known member
@Roms photo of swazi seed https://www.icmag.com/threads/swazi-red-afropips.378343/
Very beautiful seed photo my friend, hope you enjoy seeing it posted here

>>>Best>ibes
acespicoli
Well-known member
Nextgeneration73
Well-known member
Okay so I took mine out of my long term storage of 2 degrees Celcius for only a minture or so and I did so again nearly a month ago, but this time I wanted a picture for icmag, is this an issue or?Basic Points in Seed Storage
Storage Jars
Seeds require a cool and dry location in which to be best stored. Temperature and humidity fluctuations are seeds' worst enemies.
The most vigorous seeds at harvest time will keep the longest in storage. (As a principal we only sell the brands that have the most vigorous seeds.)
Improperly dried seeds can deteriorate drastically over time. (The seeds we sell have been dried properly before they are packaged and you only need to store them in a cool, low moisture environment for optimum preservation).
Bags and jars should be clearly labelled at time of storage with strain name, date and other relevant information about the strain you are preserving.
Moisture
Silica Gel Half Gram BagSeeds carry on life processes, at a low rate, whilst dormant. Moisture they absorb from the air combines with stored nourishment within the seed to form a soluble food, which then combines with oxygen from the air to release water and heat. Too much moisture in the air will cause the seed to burn up its stored food too quickly producing excess heat which will further lower the seeds ability to germinate. The need is to keep these exchanges to a minimum during storage to prolong life in the seed.
6-9% moisture is ideal for long term storage of hemp seeds. A test for moisture levels shows that hard shelled seeds like hemp seeds shatter instead of mashing at around 8% moisture when placed on concrete and struck with a hammer.
Silica gel, often used in the drying of seeds, can also be used to help maintain stable moisture levels within a permanent storage container. Equal weights of silica gel to seed are used. In general hemp seeds weigh between 0.01 and 0.02 grams and our silica gel sachets contain 0.5g. We recommend seeds are kept in aluminium zip-lock bags and stored inside seed jars along with the correct amount of silica gel to maintain low moisture levels. Be aware that you can seriously damage seeds by reducing moisture levels too much, so do not use too much dessicant. Silica gel, aluminium zip-lock bags and seed jars are all available to buy from our Seed Storage section.
Temperature
Seeds can survive temperatures that would kill the parent plant as long as they are thoroughly dried. Excess moisture in seeds that are then frozen can potentially freeze, damaging the seed.
Seeds need to be stored in a cool or cold place. Therefore, locations at floor level are preferable to those nearer the ceiling which can be significantly warmer. However, for long term storage, placing seeds in the fridge or freezer is ones best bet, as long as moisture content of the seed and storage container is low and the container is air-tight. The ideal temperature in a refrigerator is around 40F.
A freezer is best for long-term storage of seeds although you need to make sure:
You do not take the seeds out too much or for long enough for the temperature change to affect the seeds.
When you want to remove seeds from the freezer, you leave the container closed whilst the seeds warm to room temperature or otherwise condensation will form on the seeds.
Light
Similar to moisture and temperature, light can help stimulate and support the germination process. And, just as many foods, pharmaceuticals and chemicals rapidly deteriorate when exposed to light, so also is seed viability and vigour affected by being exposed to light during storage.
Seed Storage Problems
Mildew/Mould
Seeds which have not been dried to the correct moisture content before being sealed in containers, can and frequently do rot. A simple test: after "drying" and placing in closed glass jars, the appearance of condensation on the inside of the jar within a few hours indicates the need for further drying. Silica gel should help with this.
Insects
Insects that may have escaped notice can wreak havoc on stored seeds. A few pinches of diatomaceous earth (DE) is a safe, inexpensive and non-toxic way of protecting seeds against insect damage. It doesn't take much; just be sure to lightly coat all seeds before final sealing and storage. DE is available at most garden centres.
Rodents
Seeds which are not stored in glass or metal can provide a veritable banquet for mice and other small vermin. Make sure all seeds are kept in well labeled metal or glass containers.
Info provided by SM on cannabis seed storage
acespicoli
Well-known member
Biology (Basel)
. 2022 Jan 20;11(2):168. doi: 10.3390/biology11020168
Editor: Zhongqi He1
PMCID: PMC8869448 PMID: 35205035
Keywords: seed, hydration force, energy, germination, dormancy, ageing
In the absence of metabolic activity, orthodox seeds do not meet the different definitions of living organisms. The biological definition of living organisms admitted so far is based on the ability of regeneration and the existence of metabolism. NASA’s defition of life based on thermodynamic law is a “self-sustaining chemical system capable of Darwinian evolution” [4]. In both cases, living organisms can be characterized by metabolic activity having an interaction with ecological conditions. The self-sustaining chemical system corresponds to a thermodynamic aspect of life as a system far from equilibrium [5]. Yet, the seed carries the embryo ready to live as soon as the seed rehydrates. In this review, seed organization as related to water status and seed metabolism in dry state and upon imbibition are examined to understand dormancy, germination, and ageing tolerance processes.
The angiosperm seed generally consists of the embryo, the result of the fertilization of the egg cell and one of the male pollen nuclei, the endosperm, which is the result of the fusion of the two polar nuclei with the second pollen nuclei, and the perisperm, corresponding to the nucellus and the testa or seed coat formed from the integument around the ovule. The extent to which the endosperm or perisperm persists varies between species. For example, the Arabidopsis embryo is surrounded by an endosperm layer while the sunflower embryo is not (Figure 1). When the testa is underdeveloped, the outer structure being the pericarp or fruit coat, the dispersal unit is not a seed but a fruit, as in the case of sunflower and wheat. The embryo, which represents the new individual, is comprised of the embryonic axis and one or two cotyledons. The axis includes the embryonic root (radicle), the hypocotyl, and the shoot apex (plumule). Thus, as the seed corresponds to a diverse composition of such complex tissues that have distinct developmental programs [6], studying seeds implies the study of these programs and their coordination in time and space to achieve germination [7].
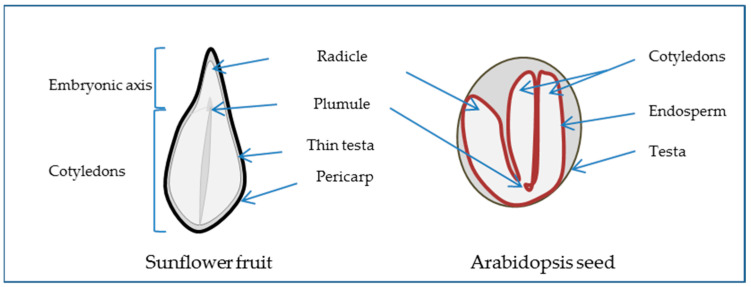
Open in a new tab
Seed morphology scheme presenting longitudinal section of sunflower fruit as a non-endospermic seed surrounded by the pericarp and Arabidopsis thaliana as an endospermic seed.
Desiccation represents the last phase of seed development and corresponds to a huge loss of water content that decreases the seed water percentage in orthodox seeds to less than 10% of the dry weight (DW), depending on species (e.g., mature sunflower seeds contain 4% g H2O/g DW [1]). Such a low water content changes the cytoplasm from a fluid to glassy state, which severely reduces molecular diffusion and mobility, preventing chemical reactions [8]. In fact, at dry state, cellular metabolism and respiration are greatly reduced [9,10]. Thus, dry seeds maintain low levels of metabolic activity, which preserves their viability for years or even centuries, as for Phoenix dactylifera L. seeds [11]. The mechanisms by which the seed tolerates desiccation are discussed in specialized reviews [12,13]. In this review, the focus will be on physiological changes allowing mature seeds to successfully undertake conservation and germination.
Seed germination starts with water uptake and ends with radicle protrusion. The seed water absorption rate corresponds to three phases during which controlled physiological processes take place. As shown in the Figure 2, phase I corresponds to a rapid water uptake, which induces the transformation of cell membranes from gel phase to liquid crystal state and the reorganization of cell structure and molecules required for the establishment of cell metabolism that takes place actively at constant water content corresponding to phase II (plateau phase), during which the water uptake is stopped. In fact, based on reports on different species, such as wheat, rice, Arabidopsis, and sunflower, phase II corresponds to high metabolic activity, with gene expression corresponding to respiration, hormones, sugar, and cell wall metabolism, and protein turn-over allowing repair and component preparation for cell elongation and growth [14,15,16,17,18]. During phase III, fast water uptake takes place again to ensure reserve mobilization and metabolism for root elongation and growth [19].
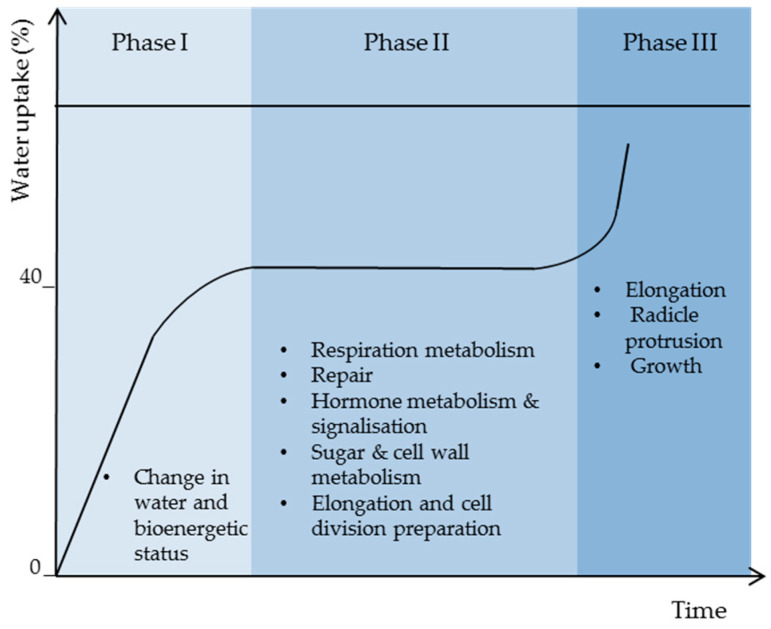
Open in a new tab
Seed imbibition curve showing the three characteristic phases with their main biological processes.
However, germination is not generally possible for mature seeds because they are generally dormant. They need to undergo a post-maturation phase, called after-ripening, a period that allows them to acquire the capacity to germinate. The transition of dry seeds from dormant (D) to non-dormant (ND) state corresponds to determinant physiological changes from arrested to permissive processes leading to germination. The characterization of possible chemical reactions and subsequent physiological activity at dry state remain the most difficult question in seed biology because experimental procedures require short- or long-term hydration. Yet, this question is crucial in the understanding of dormancy alleviation, germination, and longevity.
Thus, high water binding forces in dry seeds are responsible for the lack of stability and activity of biomolecules causing low metabolism and energy. As membrane reorganization is one of the first events in the initiation of cell energization, more water (>24%) is needed to activate protein reorganization and activity for full plasma membrane and mitochondrial energy restoration (Figure 3). Indeed, below 24% of water, seed O2 consumption is very low and it is undetectable at around 8% [9]. Respiration plays a crucial role in providing cellular energy via oxidative phosphorylation, but it also represents the major source of reactive oxygen species (ROS) responsible for cell damage. By preventing cell metabolism, drying keeps the embryo alive, which highlights the dual role of water in life and its consumption.
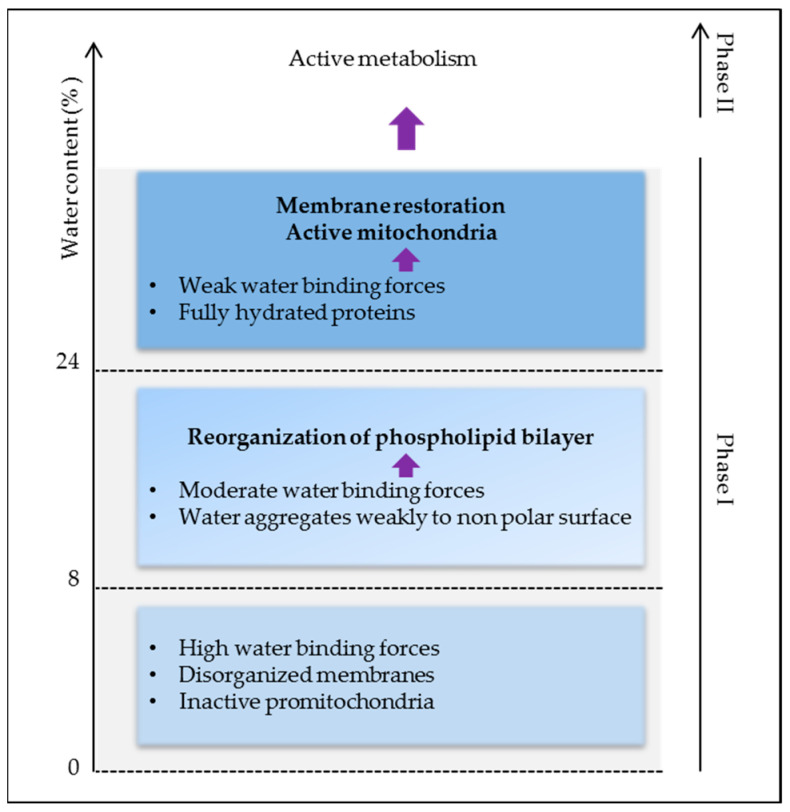
Open in a new tab
Early cellular events during imbibition as related to water binding forces.
Mitochondrial functioning is also dependent on post-translational modifications of proteins of metabolic functions. In fact, the NADPH produced from the metabolism enables the reduction of thiol redox reactions [42]. Thus, mitochondrial resumption enables not only the production of energy as ATP for elongation and growth, but also reductants that determine redox regulation for subsequent transcription and hormonal regulation.
At a water content less than 20%, the cell membrane may consist of fragments of a hexagonal array of hydrophilic circles formed by polar heads of phospholipids [43]. Such organization is responsible for electrolyte leakage and probably facilitates the massive entry of water at the beginning of imbibition. The characterization of these electrolytes in several seeds showed a great diversity of molecules, such as ions, amino-acid, sugars, organic acids, phenols, and phosphates, as well as hormones like gibberellic acid [43]. If the membrane disorganization of the dry seed inevitably induces the release of electrolytes at the beginning of imbibition, it would correspond to a powerful process which allows the seed to germinate on the poorest supports by modifying the external environment charges to create a membrane electrical potential.
In plants, plasma membrane potential is driven by two major components, K+ gradient and H+ ATPase activity. The plasma membrane (PM) H+ ATPase is responsible for membrane energization by extruding H+ protons, which is necessary for the activity of nutrient transporters associated to electrochemical H+ gradient [46]. It was demonstrated that PM H+ ATPase is essential for growth since the knockout of the two major PM H+-ATPase genes, AHA1 and AHA2, is lethal in Arabidopsis embryos [47]. The role of PM H+ ATPase in physiological processes is regulated by post-translational modifications which correspond to the phosphorylation of C terminus residues [48,49]. It was shown that PM H+-ATPase presents two activity states, auto-inhibited and upregulated, depending on the coupling ratio between ATP hydrolysis and H+ pumping [48,50,51]. The basal state has a low coupling ratio, while the activated state has a high ratio [51]. Several signals, such as sugar or light, activate the phosphorylation of C terminus, allowing the activation state corresponding to high affinity for ATP [48]. Although H+-ATPase has not been actively studied in seeds, recent work has shown that high H+-ATPase activity was associated with germination capacity while dormant state was associated with low activity in sunflower seeds [52]. Considering that the imbibition of dry seeds is driven by the physical properties of water in the reorganization and remodeling of PM, including the proper folding of H+-ATPase as a protein component, and given the central role of mitochondria and reserve mobilization, the ATP/ADP ratio of the cell may be the major parameter affecting PM H+-ATPase activity in the seed germination process. Further investigations are needed to discover the pathways by which this protein is phosphorylated and dephosphorylated in the regulation of dormancy and germination.
Non-enzymatic oxidations are possible in low hydrated seeds and represent the most plausible lead to explain the observed molecular changes reported during after-ripening [60]. Indeed, mRNA oxidation was shown to be associated with dormancy release during after-ripening in sunflower and wheat [61,62], which alters the stability of stored mRNAs, being finally degraded or translated into non-functional proteins [63]. However, if a fraction of stored mRNA is inactivated, the one involved in germination has to be protected from oxidation. A recent study showed that the association of mRNA with monosomes may be the key process for mRNA preservation [64]. The identification of translated proteins from stored mRNA in rice seeds showed that they correspond to glycolysis and translation machinery, and newly synthetized mRNA are involved in pyruvate metabolism, tricarboxylic acid (TCA) cycle, or momilactone biosynthesis [65]. This indicates that these newly synthetized energy components may represent good candidates for the regulation of germination. In fact, it was shown that TCA enzyme regulation participates in the control of seed dormancy in sunflower [16]. It was also shown that TCA enzymes were thiol redox regulated and responsible for efficient TCA functioning [41]. Several other post-translational modifications, such as phosphorylation, ubiquitination, carbonylation, glycosylation, acetylation, succinylation, or sumoylation, have been proposed to play important roles in seed germination by controlling hormonal signaling, metabolism, and redox status (for review, see [66]). Carbonylation represents the most plausible modification that takes place at dry state as a consequence of the non-enzymatic generation of reactive oxygen and nitrogen species [67]. It has been shown that protein carbonylation occurs during after-ripening and may play an important role in the transition from dormant to non-dormant state in dry seeds by facilitating reserve degradation and regulating cell signaling [68].
Respiration and redox regulation therefore constitute the most important regulation in the initiation of the germination process, but hormonal regulation that takes place later during imbibition is also crucial to germination achievement.
On the other hand, it was proposed that the ABA inhibition of growth in germinating Arabidopsis seeds is driven by its inhibitory action on PM H+-ATPase activity [77]. ABA inhibition was less effective in Arabidopsis mutants with increased capacity for H+ efflux, suggesting that cytosolic acidification due to reduced H+-ATPase activity was the main mechanism driving growth inhibition [77]. Similarly, in sunflower, ABA induced the inhibition of PM H+-ATPase in non-dormant seeds, which display hyperpolarization and subsequent membrane energization. Meanwhile, in dormant seeds, PM H+-ATPase activity was reduced even if the corresponding proteins were present and the levels of ATP were comparable to that on ND [52]. PM H+-ATPase activity is also regulated by ROS and ethylene in the opposite way [52], as proposed in the model presented Figure 4. Moreover, ND cell hyperpolarization allows sugar influx through H+/sugar symporter [52] and it was shown that glucose and fructose contents were higher in ND as compared to D seeds in the same seed model [17]. Such sugar influx along with other solutes such as K+ driven by the proton motive force of PM H+-ATPase activity could be the prerequisite for dormancy alleviation and germination as membrane energization represents the starting point for metabolism resumption by influencing water and hexose movement, but also ions and particularly protons influencing mitochondrial activity.
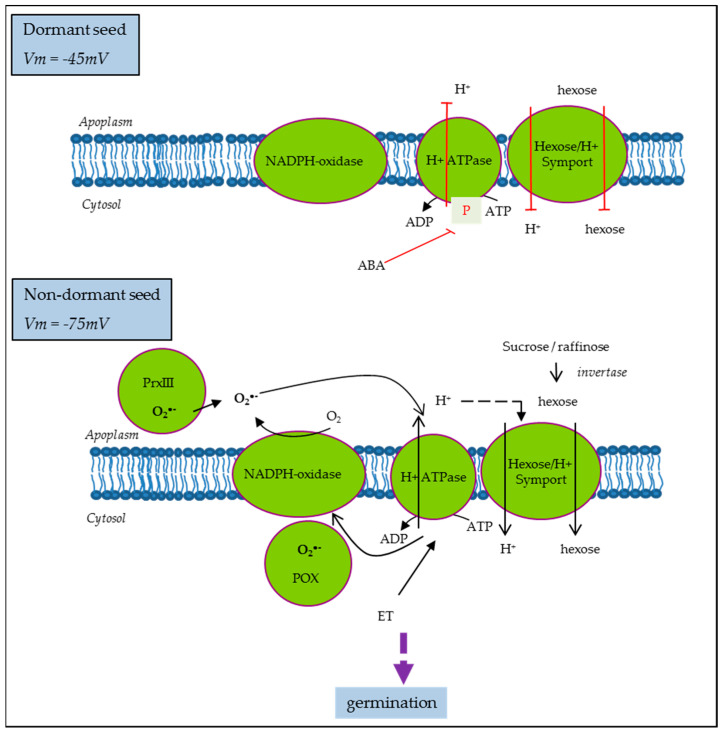
Open in a new tab
A model for seed cell polarization regulation in the control of dormancy in sunflower [52]. ABA, abscisic acid; PrxII, cell wall peroxidase III; POX, cytosolic peroxidase; ET, ethylene; Vm, PM potential.
At the scale of the whole organ, specialized tissues play a critical role upon imbibition. The most striking events are the transport of hormones from/to the different tissues of the seed and consequently their specific contribution in each tissue to regulating seed germination. For example, in endospermic seeds, ABA is produced in the endosperm and transported to the embryo, while GA goes in the opposite way [78]. Moreover, cell wall loosening and programmed cell death occur specifically in the endosperm to facilitate root protrusion [79]. In non-endospermic seeds, very scarce information is available but a recent study has shown a differentiated localization of ABA, GA, and ethylene in the meristematic zone as compared to the other parts of the seed [71]. How such tissues are differentially programmed to fulfill their respective roles and what biological structure or genetic program confers them their ability, are yet to be discovered. However, whatever the state of the seed, the signal by which it awakens comes from the environment.
Thus, environmental cues influence not only seed dormancy alleviation, but determine the depth of dormancy mediated by the mother plant during seed development and maturation. The understanding of such influence is crucial for agriculture, especially in the context of environmental condition fluctuations due to global warming.
If cellular damage is inevitably induced during ageing, the seed can resist using several features. In fact, at the cellular level, several components protect from cell damage, such as sugars, LEA, dehydrins, or heat shock proteins involved in dehydration–rehydration protection or storage proteins being preferentially oxidized protecting vital cell components from ROS damage [92]. Indeed, in the soil, seeds experience changes in temperature and water content, two major factors that influence biochemical reaction resumption, inducing ROS production and associated damages. In addition to cellular organization, the efficacy of dormancy in preventing growth resumption and the ability for damage repair are mechanisms of importance for seed longevity in natural conditions. Such repair processes are involved in the extraordinary ability of the seed to recover from ageing. They have been explored during seed priming treatment and are of interest for all living organisms.
. 2022 Jan 20;11(2):168. doi: 10.3390/biology11020168
The Seed and the Metabolism Regulation
Hayat El-Maarouf-Bouteau 1Editor: Zhongqi He1
- Author information
- Article notes
- Copyright and License information
PMCID: PMC8869448 PMID: 35205035
Abstract
Simple Summary
Seeds are the reproductive units of higher plants. They have a significant place in agriculture and plant diversity maintenance. Because they are dehydrated, they can remain viable in the environment for centuries. This review explores the dry seed as a metabolically inactive organism, but well organized to protect its components and enter intensive repair to restore metabolic activities upon imbibition for the completion of germination. Metabolism regulation is also critical for the most important seed traits, dormancy, and ageing recovery capacity.Abstract
The seed represents a critical stage in the life cycle of flowering plants. It corresponds to a dry structure carrying the plant embryo in dormant or quiescent state. Orthodox seeds possess a very low water content, preventing biochemical reactions, especially respiration. If the desiccation of living organisms leads to a loss of homeostasis, structure, and metabolism, the seeds go through it successfully thanks to their structure, cellular organization, and growth regulation. Seeds set up a certain number of sophisticated molecules to protect valuable macromolecules or organelles from dehydration/rehydration cycles. Moreover, dormancy takes place in a coordinated process with environmental cues in order to ensure embryo development at the most appropriate conditions for the establishment of the new plant. Moreover, repair processes are programmed to be ready to operate to maximize germination success and seed longevity. This review focuses on the physiology of the seed as related to hydration forces, respiration, and biochemical reactions in the transition from thermodynamically undefined dry state to self-sustained living system. Such processes are of importance for basic knowledge of the regulation of metabolism of living organisms, but also for the control of germination in the context of climate change due to global warming.Keywords: seed, hydration force, energy, germination, dormancy, ageing
1. Introduction
Seeds are important as propagation units for crops, but also for species maintenance in the natural environment. Seed germination represents the first step in the establishment of the new plant for agriculture or in natural areas. It is, therefore, important to unravel the physiological aspects of germination for basic knowledge, as well as for the good management in the context of environmental fluctuations due to global warming. Understanding germination depends on understanding the seed organization and functioning in anhydrobiosis [1]. In fact, the most important characteristic of the seeds referred to as orthodox, which are the focus of the present review, is the ability to be desiccated and to survive dry state, allowing them to be stored and distributed widely. On the contrary, recalcitrant seeds cannot tolerate dehydration. They possess a high water content and active metabolism and cannot be stored for long periods [2]. Another category with intermediate features also exists, e.g., coffee seeds, which can tolerate drying but display sensitivity to cool temperatures [3].In the absence of metabolic activity, orthodox seeds do not meet the different definitions of living organisms. The biological definition of living organisms admitted so far is based on the ability of regeneration and the existence of metabolism. NASA’s defition of life based on thermodynamic law is a “self-sustaining chemical system capable of Darwinian evolution” [4]. In both cases, living organisms can be characterized by metabolic activity having an interaction with ecological conditions. The self-sustaining chemical system corresponds to a thermodynamic aspect of life as a system far from equilibrium [5]. Yet, the seed carries the embryo ready to live as soon as the seed rehydrates. In this review, seed organization as related to water status and seed metabolism in dry state and upon imbibition are examined to understand dormancy, germination, and ageing tolerance processes.
2. Dry Seed: Well-Organized to Resist
2.1. The Seed, a Special New Individual
The seed is composed of an embryo surrounded by reserve material and covering layers. It represents the plant dispersion organ formed by sexual reproduction as well as the new individual. The seed therefore occupies a critical position in the life cycle of the higher plant. The success of the establishment of the new individual is determined by physiological and biochemical features of the seeds in response to their environment.The angiosperm seed generally consists of the embryo, the result of the fertilization of the egg cell and one of the male pollen nuclei, the endosperm, which is the result of the fusion of the two polar nuclei with the second pollen nuclei, and the perisperm, corresponding to the nucellus and the testa or seed coat formed from the integument around the ovule. The extent to which the endosperm or perisperm persists varies between species. For example, the Arabidopsis embryo is surrounded by an endosperm layer while the sunflower embryo is not (Figure 1). When the testa is underdeveloped, the outer structure being the pericarp or fruit coat, the dispersal unit is not a seed but a fruit, as in the case of sunflower and wheat. The embryo, which represents the new individual, is comprised of the embryonic axis and one or two cotyledons. The axis includes the embryonic root (radicle), the hypocotyl, and the shoot apex (plumule). Thus, as the seed corresponds to a diverse composition of such complex tissues that have distinct developmental programs [6], studying seeds implies the study of these programs and their coordination in time and space to achieve germination [7].
Figure 1.

Open in a new tab
Seed morphology scheme presenting longitudinal section of sunflower fruit as a non-endospermic seed surrounded by the pericarp and Arabidopsis thaliana as an endospermic seed.
Desiccation represents the last phase of seed development and corresponds to a huge loss of water content that decreases the seed water percentage in orthodox seeds to less than 10% of the dry weight (DW), depending on species (e.g., mature sunflower seeds contain 4% g H2O/g DW [1]). Such a low water content changes the cytoplasm from a fluid to glassy state, which severely reduces molecular diffusion and mobility, preventing chemical reactions [8]. In fact, at dry state, cellular metabolism and respiration are greatly reduced [9,10]. Thus, dry seeds maintain low levels of metabolic activity, which preserves their viability for years or even centuries, as for Phoenix dactylifera L. seeds [11]. The mechanisms by which the seed tolerates desiccation are discussed in specialized reviews [12,13]. In this review, the focus will be on physiological changes allowing mature seeds to successfully undertake conservation and germination.
Seed germination starts with water uptake and ends with radicle protrusion. The seed water absorption rate corresponds to three phases during which controlled physiological processes take place. As shown in the Figure 2, phase I corresponds to a rapid water uptake, which induces the transformation of cell membranes from gel phase to liquid crystal state and the reorganization of cell structure and molecules required for the establishment of cell metabolism that takes place actively at constant water content corresponding to phase II (plateau phase), during which the water uptake is stopped. In fact, based on reports on different species, such as wheat, rice, Arabidopsis, and sunflower, phase II corresponds to high metabolic activity, with gene expression corresponding to respiration, hormones, sugar, and cell wall metabolism, and protein turn-over allowing repair and component preparation for cell elongation and growth [14,15,16,17,18]. During phase III, fast water uptake takes place again to ensure reserve mobilization and metabolism for root elongation and growth [19].
Figure 2.

Open in a new tab
Seed imbibition curve showing the three characteristic phases with their main biological processes.
However, germination is not generally possible for mature seeds because they are generally dormant. They need to undergo a post-maturation phase, called after-ripening, a period that allows them to acquire the capacity to germinate. The transition of dry seeds from dormant (D) to non-dormant (ND) state corresponds to determinant physiological changes from arrested to permissive processes leading to germination. The characterization of possible chemical reactions and subsequent physiological activity at dry state remain the most difficult question in seed biology because experimental procedures require short- or long-term hydration. Yet, this question is crucial in the understanding of dormancy alleviation, germination, and longevity.
2.2. Water, “Matrix of Life”
If water is the matrix of life [20], dry seeds can hardly be considered as alive and yet they bear life in the form of the embryo. Water is an essential participant in the chemistry of life by sustaining the biochemistry of the cell. It acts as a liquid and solvent for biochemical reactions, but also influences macromolecule structures [21]. Water participates in the catalytic function of proteins and nucleic acids and physically in hydrophobic associated protein folding and complex formation through the hydrogen bond [22]. Physical methods, such as thermodynamic studies or nuclear magnetic resonance spectroscopy, came to the rescue of biology for the investigation of water status in low hydrated seeds and subsequent interactions. Using thermodynamic measurement, three levels of water affinity have been characterized in pea and soybean seeds [9,23,24]. Strongly bound water was recorded at 8% of water content, weakly bound water between 8% and 24%, and very loosely bound water at contents above 24% [9]. In these ranges of water moisture, the investigation of lysozyme hydration by IR spectroscopy and heat capacity showed that with up to 0.07 g of water/g of protein, the hydration process is dominated by the interaction with charged groups. At 0.07 g/g, there is a transition in the IR spectrum and the heat capacity, reflecting a change in surface water arrangements. Between 0.07 and 0.25 g/g, most of the surface is covered with water molecules. Between 0.25 and 0.38 g/g, water condenses over the non-polar atoms not adjacent to charged or polar atoms [25]. The final stage of protein hydration is that of hydrophobic groups, which represent a large portion of the surface of the protein molecule. Water–water bonds can be then created and participate in protein–protein or protein–substrate interactions. The enzymatic activity of lysozymes becomes detectable at 0.2 g/g and changes with hydration above 0.38 g/g. Changes in the arrangement of water in the protein environment affect protein stability and enzyme properties [25,26]. On the other hand, nucleic acids require more water than proteins [25]. In fact, the end point of the hydration process of nucleic acids is about twice the level for proteins [27]. DNA structure and related biological functions are controlled by the complex dynamics of hydrating water and ions in and around the DNA [28]. It was shown that in desiccated Arabidopsis seeds the chromatin is highly condensed and can be de-condensated after hydration [29]. The property of the seed to undergo a reversible chromatin condensation/de-condensation enables to withstand desiccation and the entry in active metabolism during imbibition.Thus, high water binding forces in dry seeds are responsible for the lack of stability and activity of biomolecules causing low metabolism and energy. As membrane reorganization is one of the first events in the initiation of cell energization, more water (>24%) is needed to activate protein reorganization and activity for full plasma membrane and mitochondrial energy restoration (Figure 3). Indeed, below 24% of water, seed O2 consumption is very low and it is undetectable at around 8% [9]. Respiration plays a crucial role in providing cellular energy via oxidative phosphorylation, but it also represents the major source of reactive oxygen species (ROS) responsible for cell damage. By preventing cell metabolism, drying keeps the embryo alive, which highlights the dual role of water in life and its consumption.
Figure 3.

Open in a new tab
Early cellular events during imbibition as related to water binding forces.
2.3. Respiration Resumption
Holding respiration may represent the major process allowing seed longevity. This is achieved with the support of several seed features. The seed structure itself may contribute to holding respiration by O2 uptake limitation due to the space occupation by reserve molecules and the presence of the seed coat (for review [13,30]). More importantly, mitochondria in dry seeds are called promitochondria as their internal membranes are underdeveloped with a low number of cristae and low protein content [31,32]. Several studies using different biochemical approaches, such as adenylate pool or adenylate energy charge (AEC) ratio ((ATP + 0.5 ADP)/(AMP + ADP + ATP)), oxygen uptake, tricarboxylic cycle enzyme activity measurements, or cytological investigations, converge to state that respiration is reduced to a very low level in dry seeds and that the hydration induces an increase in mitochondria components and activity [33,34,35,36,37,38,39]. The proliferation and differentiation of mitochondria, called ‘mitochondrial biogenesis’, occur progressively upon imbibition [10,37,38]. These are considered as the prerequisite for the full reactivation of mitochondria and subsequent energy supply for germination [31,32]. However, isolated promitochondria were shown to be able to generate ATP and a membrane potential by oxidizing supplied succinate and/or NADH [31,40]. Such metabolic activity may be decisive at the onset of imbibition to help the biogenesis process to take place. Moreover, promitochondria seem to have an import apparatus ready for mitochondrial biogenesis [32]. In fact, the electron transport system is activated immediately after the initiation of imbibition and is dependent on AMP, ADP, cytochrome C oxidase, and ATPase that were recovered from dry seeds [36,41]. A recent study enabled the visualization of mitochondrial reactivation and the chondriome (all mitochondria in a cell) during imbibition [10]. They confirmed that promitochondria have reduced metabolic activity but can generate a membrane potential within the first minutes of imbibition. Further imbibition in permissive conditions for germination allowed a significant increase of mitochondrial dynamics, leading to inter-mitochondria interactions and localization around the nucleus, which may facilitate mitochondrial biogenesis and synchronization [10].Mitochondrial functioning is also dependent on post-translational modifications of proteins of metabolic functions. In fact, the NADPH produced from the metabolism enables the reduction of thiol redox reactions [42]. Thus, mitochondrial resumption enables not only the production of energy as ATP for elongation and growth, but also reductants that determine redox regulation for subsequent transcription and hormonal regulation.
2.4. Plasma Membrane Potential
One of the fundamental properties of living cells is the establishment of an electrical potential difference across the plasma membrane. In dry seeds, the transport of ions is not possible due to the absence of water as a conductive fluid, but also due to the loss of the integrity of membranes and their protein components. Membrane deterioration has been highlighted by the high electrolyte leakage rate measured in dry seeds and many studies have reported that seed hydration induced a membrane leakage decrease, confirming that the cell membrane was repaired upon imbibition. It was shown that the leakage decrease depends on the moisture content of the seeds, being undetectable above 24% [9]. In fact, 20% water corresponds to the minimum amount of water needed to create a hydrophilic layer that stabilizes the organization of lipids in a bilayer [43]. Such a water content corresponds to phase I of germination (Figure 3), while lipid metabolism and repair were shown to take place during phase II [44,45], suggesting the need for an intact membrane before any repair event can be started. It also corresponds to the start point of the full hydration of proteins (discussed above) that should include membrane ion channels and transporters.At a water content less than 20%, the cell membrane may consist of fragments of a hexagonal array of hydrophilic circles formed by polar heads of phospholipids [43]. Such organization is responsible for electrolyte leakage and probably facilitates the massive entry of water at the beginning of imbibition. The characterization of these electrolytes in several seeds showed a great diversity of molecules, such as ions, amino-acid, sugars, organic acids, phenols, and phosphates, as well as hormones like gibberellic acid [43]. If the membrane disorganization of the dry seed inevitably induces the release of electrolytes at the beginning of imbibition, it would correspond to a powerful process which allows the seed to germinate on the poorest supports by modifying the external environment charges to create a membrane electrical potential.
In plants, plasma membrane potential is driven by two major components, K+ gradient and H+ ATPase activity. The plasma membrane (PM) H+ ATPase is responsible for membrane energization by extruding H+ protons, which is necessary for the activity of nutrient transporters associated to electrochemical H+ gradient [46]. It was demonstrated that PM H+ ATPase is essential for growth since the knockout of the two major PM H+-ATPase genes, AHA1 and AHA2, is lethal in Arabidopsis embryos [47]. The role of PM H+ ATPase in physiological processes is regulated by post-translational modifications which correspond to the phosphorylation of C terminus residues [48,49]. It was shown that PM H+-ATPase presents two activity states, auto-inhibited and upregulated, depending on the coupling ratio between ATP hydrolysis and H+ pumping [48,50,51]. The basal state has a low coupling ratio, while the activated state has a high ratio [51]. Several signals, such as sugar or light, activate the phosphorylation of C terminus, allowing the activation state corresponding to high affinity for ATP [48]. Although H+-ATPase has not been actively studied in seeds, recent work has shown that high H+-ATPase activity was associated with germination capacity while dormant state was associated with low activity in sunflower seeds [52]. Considering that the imbibition of dry seeds is driven by the physical properties of water in the reorganization and remodeling of PM, including the proper folding of H+-ATPase as a protein component, and given the central role of mitochondria and reserve mobilization, the ATP/ADP ratio of the cell may be the major parameter affecting PM H+-ATPase activity in the seed germination process. Further investigations are needed to discover the pathways by which this protein is phosphorylated and dephosphorylated in the regulation of dormancy and germination.
3. Seed Dormancy: Higher Level of Resistance
Seed dormancy, which is the incapacity of mature seeds to germinate, is one of the most important processes in the successful establishment of the new seedling. Dormancy is finely regulated with the aim to insure germination at the optimal moment. Indeed, deep dormancy prevents field emergence and low dormancy causes sprouting. Dormancy takes place at the end of seed formation, and it’s removed during a period of several weeks to decades, called after-ripening.3.1. Seed Metabolism and Dormancy
After-ripening has fascinated researchers because dormancy is alleviated in dry conditions, suggesting that some processes operate in the dry seed. Biological reactions have been investigated and transcriptional programs have been proposed to be involved in the regulation of after-ripening-mediated seed dormancy alleviation in several seeds [53,54,55,56,57]. Given the restricted molecular mobility due to the glassy state in dry seed cells, the existence of a hydrated pocket within the cell enabling gene transcription has been hypothesized [53]. To address this issue, Meimoun et al. [1] investigated transcriptomic changes after the after-ripening period in sunflower seeds using two protocols, one allowing dormancy alleviation but not the other, in order to differentiate between changes in gene expression associated with dormancy alleviation and those associated with storage only. They showed that there is no significant variation between conditions, suggesting that gene expression did not take place during after-ripening, in agreement with the absence of metabolic activity in dry seeds [1]. Furthermore, ancient studies (over 50 years) have already shown that transcription was not required for de novo protein synthesis in imbibed seeds, suggesting that seeds contain stored transcripts ready for use upon imbibition [58]. Since then, a number of studies have demonstrated that germination (reaching radicle protrusion) is completed in the presence of a transcription inhibitor while it is completely blocked in the presence of a translation inhibitor (for review, see [59]). This means that stored mRNAs, also called ‘long-lived mRNAs’, are necessary and sufficient to carry out the germination in sensu stricto which corresponds to the determining phase of dormancy maintenance or alleviation.Non-enzymatic oxidations are possible in low hydrated seeds and represent the most plausible lead to explain the observed molecular changes reported during after-ripening [60]. Indeed, mRNA oxidation was shown to be associated with dormancy release during after-ripening in sunflower and wheat [61,62], which alters the stability of stored mRNAs, being finally degraded or translated into non-functional proteins [63]. However, if a fraction of stored mRNA is inactivated, the one involved in germination has to be protected from oxidation. A recent study showed that the association of mRNA with monosomes may be the key process for mRNA preservation [64]. The identification of translated proteins from stored mRNA in rice seeds showed that they correspond to glycolysis and translation machinery, and newly synthetized mRNA are involved in pyruvate metabolism, tricarboxylic acid (TCA) cycle, or momilactone biosynthesis [65]. This indicates that these newly synthetized energy components may represent good candidates for the regulation of germination. In fact, it was shown that TCA enzyme regulation participates in the control of seed dormancy in sunflower [16]. It was also shown that TCA enzymes were thiol redox regulated and responsible for efficient TCA functioning [41]. Several other post-translational modifications, such as phosphorylation, ubiquitination, carbonylation, glycosylation, acetylation, succinylation, or sumoylation, have been proposed to play important roles in seed germination by controlling hormonal signaling, metabolism, and redox status (for review, see [66]). Carbonylation represents the most plausible modification that takes place at dry state as a consequence of the non-enzymatic generation of reactive oxygen and nitrogen species [67]. It has been shown that protein carbonylation occurs during after-ripening and may play an important role in the transition from dormant to non-dormant state in dry seeds by facilitating reserve degradation and regulating cell signaling [68].
Respiration and redox regulation therefore constitute the most important regulation in the initiation of the germination process, but hormonal regulation that takes place later during imbibition is also crucial to germination achievement.
3.2. Internal Determinants of Dormancy
It is well established that dormancy is regulated by the hormonal balance between the main positive regulator abscisic acid (ABA) and negative ones, such as hormones like gibberellic acid (GA), ethylene (ET), auxins, or brassinosteroids, as well as some other molecules, like ROS or nitric oxide (NO). The involvement of each of them and their interactions in the whole process of germination depend on the structure of the seed and the environment. Nevertheless, ABA represents the highly conserved component of the process across species and the unique dormancy determinant as opposed to the multiple stimulants of germination. Thus, to illustrate the regulation and function of hormones in the physiology of germination, without elaborating on all the hormones and their complex signaling, the case of the ABA is the most appropriate. High ABA is induced during the maturation phase of seed development to set up desiccation and dormancy. In mature seeds, a large proportion of the stored mRNA was shown to correspond to genes in which the promoters are targets of ABA-responsive transcription factors, which could be the residual consequence of the ABA induction in the maturation phase or a regulated process to insure the execution of ABA signaling upon imbibition [69]. Upon imbibition, ABA content declines similarly in ND and D seeds during the early phase of germination, but this decrease continues in ND seeds while subsequent de novo ABA synthesis occurred in imbibed D seeds, leading to dormancy maintenance [70]. Therefore, ABA biosynthesis, catalysis enzymes, and corresponding genes, nine-cis-epoxycarotenoid dioxygenase (NCED) and cytochrome P450 707A (CYP707A), respectively, represent the major determinants of seed dormancy. However, a decrease in ABA content is not a prerequisite for germination as ABA signaling events represent another level of regulation [71]. The responsiveness of seeds to ABA is called ABA sensitivity and it involves several promoters, genes, and protein regulations. In recent studies, a number of these key players have been characterized in a complex network partly connected with other hormones having a dual role in this process [72,73]. However, how such players operate to arrest expansion of the embryo and growth remains unsolved. Considering the challenging energy demand of the germination process, reserve breakdown and respiration may represent the regulatory mechanisms. ABA treatment is able to inhibit reserve mobilization and sugar treatment to overcome the exogenous ABA inhibition of germination. However, the effect of endogenous ABA is still unclear [74]. Mitochondria play a central role in energy supply and they are also associated with ABA sensitivity based on works showing that several mutants of RNA processing for subunits of the electron chain display reduced ABA sensitivity. This regulation involves retrograde, anterograde, and inter-organelle signals in the transcription control of the ABA biosynthesis gene, NCED [75]. On the other hand, Paszkiewicz et al. [10] have shown that mitochondrial dynamics associated with germination condition was slightly affected by ABA treatment, arguing that mitochondrion reactivation depends only on the physical conditions of hydration and temperature. Based on these works, the optimal differentiation and functioning of mitochondrion are associated with an ABA sensitivity decrease. Accordingly, it is easy to consider that in dormant seeds, the impairment of mitochondrial activity occurs. However, it has long been established that inhibitors of oxidative phosphorylation such as cyanid can break dormancy. This paradox has still not been elucidated. The activation of the pentose phosphate pathway, the metabolic pathway that supplies reducing energy to cells, has been the most plausible hypothesis proposed [76]. Indeed, in reduced mitochondrial activity, glycolysis is activated to obtain ATP, a phenomenon known the “Pasteur effect”, leading to pyruvate production and the accumulation of fermentation by-products. Thus, anaerobic metabolism facilitates reserve breakdown and it might operate, in normal conditions, at the onset of germination when the mitochondria are not yet fully reactivated. All these data point to the importance of cell metabolism and energy regulation for successful germination.On the other hand, it was proposed that the ABA inhibition of growth in germinating Arabidopsis seeds is driven by its inhibitory action on PM H+-ATPase activity [77]. ABA inhibition was less effective in Arabidopsis mutants with increased capacity for H+ efflux, suggesting that cytosolic acidification due to reduced H+-ATPase activity was the main mechanism driving growth inhibition [77]. Similarly, in sunflower, ABA induced the inhibition of PM H+-ATPase in non-dormant seeds, which display hyperpolarization and subsequent membrane energization. Meanwhile, in dormant seeds, PM H+-ATPase activity was reduced even if the corresponding proteins were present and the levels of ATP were comparable to that on ND [52]. PM H+-ATPase activity is also regulated by ROS and ethylene in the opposite way [52], as proposed in the model presented Figure 4. Moreover, ND cell hyperpolarization allows sugar influx through H+/sugar symporter [52] and it was shown that glucose and fructose contents were higher in ND as compared to D seeds in the same seed model [17]. Such sugar influx along with other solutes such as K+ driven by the proton motive force of PM H+-ATPase activity could be the prerequisite for dormancy alleviation and germination as membrane energization represents the starting point for metabolism resumption by influencing water and hexose movement, but also ions and particularly protons influencing mitochondrial activity.
Figure 4.

Open in a new tab
A model for seed cell polarization regulation in the control of dormancy in sunflower [52]. ABA, abscisic acid; PrxII, cell wall peroxidase III; POX, cytosolic peroxidase; ET, ethylene; Vm, PM potential.
At the scale of the whole organ, specialized tissues play a critical role upon imbibition. The most striking events are the transport of hormones from/to the different tissues of the seed and consequently their specific contribution in each tissue to regulating seed germination. For example, in endospermic seeds, ABA is produced in the endosperm and transported to the embryo, while GA goes in the opposite way [78]. Moreover, cell wall loosening and programmed cell death occur specifically in the endosperm to facilitate root protrusion [79]. In non-endospermic seeds, very scarce information is available but a recent study has shown a differentiated localization of ABA, GA, and ethylene in the meristematic zone as compared to the other parts of the seed [71]. How such tissues are differentially programmed to fulfill their respective roles and what biological structure or genetic program confers them their ability, are yet to be discovered. However, whatever the state of the seed, the signal by which it awakens comes from the environment.
3.3. Environmental Impact on Dormancy
Environmental factors are of high importance in the awakening of the seed. Their effect on seed performance was shown to surpass genetic impact [80]. Temperature and soil moisture oscillations are the major players under natural conditions. Indeed, alternating temperatures more than constant ones can promote germination via the interplay between ROS signaling and hormones [81]. In fact, it has been shown that fluctuating temperatures alleviate dormancy by reducing ABA synthesis and signaling [82]. In the absence of a change in ABA content, there was a decrease in ABA sensitivity in sunflower dormant seeds in response to constant temperature that induced dormancy alleviation [71]. The activity of several enzymes of TCA and glycolysis were shown to be altered in the same model [16]. In wheat, high temperature treatment during seed development affects mitochondrion functioning by reducing the respiration rate and ATP content [83]. Indeed, elevated temperatures experienced by the mother plant during seed development and maturation had a negative effect on seed composition, germination, and vigor (for review, see [84]). On the other hand, light and nitrate also play important roles. Their effects are associated in dormancy cycling [85,86,87]. Seed sensitivity to both of them depends on the season and depth of dormancy. A low concentration of nitrate (around 0.1 mM) is able to promote seed germination in several species [85]. Several evidences converge towards nitrate induction of CYP707A2 leading to ABA decrease more than GA biosynthesis in dormancy breakdown [85]. However, GA biosynthesis gene involvement has also been reported in response to environmental cues in Arabidopsis seeds from lab but also soil seed bank experiences [88,89]. Moreover, the analysis of the whole transcriptome change by nitrate treatment during seed imbibition showed the upregulation of genes involved in nitrate assimilation and transport, hormone metabolism, and energy, such as Glucose-6-phosphate dehydrogenase2, highlighting the importance of the pentose phosphate pathway [90]. Interestingly, at the level of gene expression, different environmental signals, such as light, nitrate, stratification, or after-ripening induced common changes associated to dormancy release [88]. They concern genes belonging to translation machinery, cell wall modification, and reserve mobilization. Such changes in transcript abundance are reversible, allowing the dormancy cycling phenomenon which occurs on entering a secondary dormancy when unfavorable environmental conditions are prolonged after the primary dormancy alleviation. In fact, dormancy is tightly regulated in natural conditions as in the soil, when seeds experience several scenarios of temperature, light, nitrate, and moisture, as well as microbial environment. The latter corresponds to a wide range of microbes, as pathogenic ones can induce decrease in seed longevity due to infection, and others can influence seed dormancy by breaking down the seed coat [12,91].Thus, environmental cues influence not only seed dormancy alleviation, but determine the depth of dormancy mediated by the mother plant during seed development and maturation. The understanding of such influence is crucial for agriculture, especially in the context of environmental condition fluctuations due to global warming.
4. Seeds: The Ability to Recover from Ageing
Seeds remain viable, i.e., capable to germinate producing a viable plantlet, generally for a long period, from weeks to thousands of years, depending on species. Seed longevity is important for economic aspects of trade and agronomy associated with storage, but also for maintaining biodiversity. Obviously, reduced water content and metabolic activity enable such great longevity. However, long-term conservation results in a loss of viability due to deterioration processes. More specifically, unsuitable conditions of conservation, such as temperature and moisture, experienced by seeds in natural conditions or during storage accelerate deterioration processes, such as the loss of membrane integrity and oxidation of macromolecules, leading to the impairment of metabolism. However, the seed has the extraordinary ability to recover using extensive repair machinery, which represents another performance to stay alive.4.1. Seed Ageing
Seed life span is of importance for field crop species impacting agriculture, but also for plant species diversity maintenance by its impact on seed longevity in the soil [92]. Seed ageing was defined as the loss of seed quality and viability over time [93]. Aged seeds germinate poorly giving abnormal seedlings or ultimately are unable to germinate. Orthodox seeds are resistant to ageing for very long time because they have a very low water content, resulting in reduced cell metabolism especially respiration which is responsible for the major production of ROS [94]. Indeed, as for the dry after-ripening process described above, enzymatic reactions and respiration are restricted by the lack of free available water preventing cellular damage. It was shown that long term storage resulting in seed loss of viability is associated with the impairment of mitochondrial activity and protein synthesis machinery [95,96]. Mitochondrion membrane integrity was identified as the primary target for ageing leading to the deregulation of its oxidative properties [97]. ROS are considered as the major cause of seed deterioration due to the oxidation of its components [98]. Lipid peroxidation has been reported in several studies on different seed species, influencing lipid metabolism and membrane integrity, as for sunflower seeds [99,100]. Furthermore, DNA damage producing double- or single- strand breaks or damaged bases is responsible for genome integrity loss and subsequent low seed quality [101]. DNA laddering has been shown in sunflower and pea aged seeds when damage extent exceeded repair capacity, pointing out the key role of mitochondria dysfunction in seed ageing [102,103]. Indeed, a direct correlation between ROS production and mitochondrial impairment leading to programed cell death has been shown in Ulmus pumila L. [104]. At last, total RNA content and integrity was shown to decrease in aged soybean seeds, with greater resistance of the shortest transcript (<1200 bp) mainly involved in ribosomal and translational functions, as compared to the longer transcripts (>2500 bp) corresponding to proteins with ATP-binding functions, indicating that stored mRNAs may be involved in seed longevity [105,106].If cellular damage is inevitably induced during ageing, the seed can resist using several features. In fact, at the cellular level, several components protect from cell damage, such as sugars, LEA, dehydrins, or heat shock proteins involved in dehydration–rehydration protection or storage proteins being preferentially oxidized protecting vital cell components from ROS damage [92]. Indeed, in the soil, seeds experience changes in temperature and water content, two major factors that influence biochemical reaction resumption, inducing ROS production and associated damages. In addition to cellular organization, the efficacy of dormancy in preventing growth resumption and the ability for damage repair are mechanisms of importance for seed longevity in natural conditions. Such repair processes are involved in the extraordinary ability of the seed to recover from ageing. They have been explored during seed priming treatment and are of interest for all living organisms.
4.2. Seed Priming
Seeds possess effective repair machinery to cope with ageing-associated oxidative damage. Several non-enzymatic antioxidants have been proposed to be determinant in seed longevity, e.g., glutathione or ascorbic acid [107,108]. The antioxidant enzymes are also of importance in ROS detoxification, such as catalase, superoxide dismutase, ascorbate peroxidase, or glutathione reductase [109,110]. Other enzymes acting on specific macromolecules are also activated, such as DNA or protein repair enzymes [101,111,112]. Such machinery operates when seed hydration occurred, and its efficiency depends on the plant species and the extent of ageing damage. Based on this feature, a priming technique has been developed to improve seed quality from alterations caused by several stresses. Priming treatment consists of seed pre-hydration with a controlled amount of water which does not allow radicle elongation, i.e., a water amount corresponding phase II of the germination sensu stricto, which is sufficient to trigger the reparation processes. The addition of beneficial molecules, such as antioxidants or hormones, during priming treatment can further increase seed reparation and subsequent quality. Several priming techniques have been developed depending on the plant species and subsequent use. They all lead to an improvement of seed performance under variable environmental conditions [113]. It was shown that repair mechanisms and oxidative management in primed seeds represent the main processes associated with priming induced germination improvement, but DNA replication, cell cycle advancement, the modification of the membrane structure, and restoration of mitochondrial integrity were also proposed to explain the priming effect in germination improvement [114]. In fact, the seed engages growth preparation processes during imbibition while maintaining the desiccation tolerance machinery which allowed a successful dehydration after the priming treatment. The dried primed seed is ready to grow better, even under stress conditions. This feature is interesting for agriculture, which is why the priming technique is widely used. Priming may be even more useful in the years to come due to changing climate conditions.5. Conclusions
Understanding the successful entry and exit from desiccation is fundamental for the improvement of seed germination under challenging conditions anticipated due to global warming. The application in plant germplasm conservation in seed banks is of high importance in the maintenance of genetic resources for food and environment security. In this review, several layers of regulations of seed performance were shown, from the organization and physical protection of cell components to the regulation of several signaling processes in a coordinated crosstalk. However, their implementation and the coordination of these mechanisms during seed development deserve more investigations.acespicoli
Well-known member
Okay so I took mine out of my long term storage of 2 degrees Celcius for only a minture or so and I did so again nearly a month ago, but this time I wanted a picture for icmag, is this an issue or? View attachment 19071773
| Formula | (2°C × 9/5) + 32 = 35.6°F |
Id leave them in the fridge as much as possible, a quick peek once or twice. You should be good!

acespicoli
Well-known member
Metabolic Processes During Seed Germination
WRITTEN BYAwatif S. Ali and Alaaeldin A. Elozeiri
Reviewed: 21 August 2017 Published: 06 December 2017
DOI: 10.5772/intechopen.70653

Seed BiologyEdited by Jose C. Jimenez-Lopez
FROM THE EDITED VOLUME
Advances in Seed Biology
Edited by Jose C. Jimenez-LopezMetabolic Processes During Seed Germination | IntechOpen
Abstract
Seed germination is crucial stage in plant development and can be considered as a determinant for plant productivity. Physiological and biochemical changes followed by morphological changes during germination are strongly related to seedling survival rate and vegetative growth which consequently affect yield and quality. This study is aimed to focus on proceeding of the most vital metabolic processes namely reserve mobilization, phytohormonal regulation, glyoxylate cycle and respiration process under either stressful or non-stressful conditions that may be led to suggest and conduct the more successful experimental improvements. Seed imbibition triggered the activation of various metabolic processes such as synthesis of hydrolytic enzymes which resulted in hydrolysis of reserve food into simple available form for embryo uptake. Abiotic stresses potentially affect seed germination and seedling establishment through various factors, such as a reduction in water availability, changes in the mobilization of stored reserves, hormonal balance alteration and affecting the structural organization of proteins. Recent strategies for improving seed quality involved classical genetic, molecular biology and invigoration treatments known as priming treatments. H2O2 accumulation and associated oxidative damages together with a decline in antioxidant mechanisms can be regarded as a source of stress that may suppress germination. Seed priming was aimed primarily to control seed hydration by lowering external water potential, or shortening the hydration period.Keywords
- reserve mobilization
- proteolysis
- glyoxylate cycle
- phytic acid
- seed priming
- stress tolerance mechanisms
Author Information
Show +1. Introduction
Seed germination is vital stage in plant development and can be considered as a determinant for plant productivity. It begins by water imbibition, mobilization of food reserve, protein synthesis and consequence radicle protrusion [1]. To sustain a good seedling development, seed stores a food reserve mainly as proteins, lipids and carbohydrates [2]. Protein and oil bodies are the major reserve in oilseed which represent a source for each of energy, carbon, and nitrogen during seedling establishment [3]. Because the physiology of reserve mobilization during germination and post-germination events is still poorly understood, extensive studies must be performed to know the metabolic mechanisms of reserve food mobilization providing insights into the ability to use such seeds as planting material [4]. Enzymatic hydrolysis of protein, lipid and carbohydrate, and transportation of metabolites is dependent mainly on water availability [5].Physiological and biochemical changes followed by morphological changes during germination are strongly related to seedling survival rate and vegetative growth which affect yield and quality. Food reserve of starch and protein are mainly stored in the endosperm. In general, germination process can be distinguished into three phases: phase I, rapid water imbibition by seed; phase II, reactivation of metabolism; and phase III, radicle protrusion [6]. The most critical phase is phase II whereas, the essence physiological and biochemical processes such as hydrolysis, macromolecules biosynthesis, respiration, subcellular structures, and cell elongation are reactivated resulting in initiation of germination [7].
Water imbibition by reserve substances in germinating wheat seed stimulates the embryo to produce phytohormones mainly gibberellic acid (GA) which can diffuse to aleurone layer and initiate a signaling cascade resulting in the synthesis of α-amylases and other hydrolytic enzymes. Then, hydrolytic enzymes secrete into the endosperm and hydrolyzed food reserve [8, 9]. Germination is considered a response includes bidirectional interactions between the embryo and endosperm since the endosperm can secrete signals to control embryo growth [10]. Previous studies were investigated the activity of some key enzymes in glycolysis, pentose phosphate pathway (PPP), the tricarboxylic acid cycle (TCA cycle), and amino acid metabolism during germination [11].
Seed germination is particularly vulnerable to environmental stress encountered conditions, specifically salt and water which are widespread problem around the world [12]. High salt and drought tolerance seeds might be showed rapid germination resulting in a good seedling establishment and hence expected to maintain high yield productivity [13]. Water and salt stress conditions affect seed germination with reducing germination rate and delay in the initiation of germination [14]. Under water stress, enzymes activity such as α-amylase in Cicer arietinum cotyledons [15] or α- and β-amylase in Medicago sativa germinating seeds [16] were reduced. In contrast, water stress conditions led to an increase in the activity of α-amylase in Hordeum vulgare seedlings [17], β-amylase in Cucumis sativus cotyledons [18], cytosolic glyceraldehyde-3-phosphate dehydrogenase in Craterostigma plantagineum plants [19] and protease in Oryza sativa seedlings [20]. Salt stress causes ion toxicity, osmotic stress and reactive oxygen species (ROS) stress [21]. ROS reacts with cell macromolecules [22] and lipids [23], and disrupt diverse physiological and biochemical processes, such as hormonal imbalance and reduced use of reserves [24]. Plants develop ROS-scavenging mechanisms include enzymatic and non-enzymatic antioxidant systems [25] that protect plants against oxidative damage. Therefore, improvement the activity of antioxidant enzymes in plants organs is necessary for increasing plant’s salt tolerance. Species and varieties/cultivars varied in their ability for salt tolerance mechanism. Comparing with adult plant, the mechanisms of stress tolerance in germinating phase are poorly interpreted and might be related to a series of factors that are inherent to the species and environment [26, 27].
Phytohormones have essential role in inducing plant acclimatization to change in environmental conditions by mediating growth, development, source/sink transitions, and nutrient allocation [28]. Phytohormones are considered the most important endogenous substances for modulating physiological and molecular responses [28]. They include auxin (IAA), cytokinins (CKs), abscisic acid (ABA), ethylene (ET), gibberellins (GAs), salicylic acid (SA), brassinosteroids (BRs), and jasmonates (JAs). The strigolactone (SL) are relatively new phytohormones.
Genetically and physiological studies have been demonstrated the effective roles of the plant hormones ABA and GAs in regulation of dormancy and germination [29]. To counteract the adverse effects of abiotic stress, seed priming methods have been applied to improve germination, uniformity, improve seedling establishment and stimulate vegetative growth in more field crops [30, 31]. Wheat seeds were priming to increase germination characteristics and stress tolerance. As seeds imbibe water, metabolic processes initiate with an increase in respiration rate [7]. Early developmental stages of seedling require fueling energy before it becomes autotrophic [32].
Seeds store mineral nutrients as sucrose or amino acids which are synthesized into starch or proteins during development to be used in early seedling emergence. Phosphorus is taken up by plants as phosphate and translocate to developed seeds where it is stored in phytic acid form mainly (about 75%).
2. The role of hydrolytic enzymes in seed germination
On seed hydration, separate intercellular bodies of seed stored carbohydrates, proteins, lipid and phosphate act as energy source and carbon skeleton [33]. Seed imbibition triggered many metabolic processes such as activation or freshly synthesis of hydrolytic enzymes which resulted in hydrolysis of stored starch, lipid, protein hemicellulose, polyphosphates and other storage materials into simple available form for embryo uptake. Also, consumption of an elevated level of oxygen may be induced activation/hydration of mitochondrial enzymes, involved in the Krebs cycle and electron transport chain [34, 35].2.1. Hydrolysis of storage seed proteins
Proteolytic enzymes have the main role in using stored protein in metabolism of germinating seeds which proceed through many stages [36]. According to Gepstin and Ilan [37], proteolytic activity in germinating beans increased during the first 7 days which partially dependent on the embryonic axis. Proteases and peptidases have been detected in many seeds during germination whereas; plant protease and amylase inhibitors which are proteinaceous in nature are being disappeared [38]. Antitryptic and antichymotryptic activities were observed to be markedly reduced in the endosperm of finger millet on germination which might be attributed to the proteolytic activity in hydrolysis of the inhibitory proteins [39]. Hydrolysis of stored proteins produced free amino acids, which support protein synthesis in endosperm and embryo and so proceeding of germination process [40]. Schlereth et al. [41] recorded an initial little decrease in free amino acids at the beginning of vetch seeds imbibition which is attributed to leakage from the axis, but remain without change during late germination stage.A disulfide proteome technique was developed by Yano et al. [42] to visualize redox changes in proteins. This technique was used to analyze rice bran resulting in identification of embryo-specific protein 2 (ESP2), dienelactone hydrolase, putative globulin, and globulin-1S-like protein as putative target of thioredoxin, which support the hypothesis that thioredoxin activates cysteine protease with a concurrent unfolding of its substrate during germination [43].
In buckwheat seeds, the main storage protein constituent about 16% of total seed protein is the 13S globulin with molecular mass of about 300 kDa and consists of acid and basic subunits with molecular masses ranging from 57.5 to 23.5 kDa [44]. During seed germination, 13S globulin is hydrolyzed by proteolytic enzymes through stages and the products are used by the growing seedling. The first stage of the 13S globulin degradation resulted from a limited proteolysis activity of metalloproteinase with the cleavage of about 1.5% of peptide bonds. This stage proceeds during the first 3 days of germination. It takes place during the first 3 days of germination [45]. Metalloproteinase activity is controlled by a proteinaceous inhibitor (Mr—10 kDa), present in dry buckwheat seeds in a complex with the enzyme which dissociated by bivalent cations liberated from phytin hydrolysis process. Phytin is present in buckwheat seeds in sufficient amount in the form of globoids disposed in protein bodies [46].
During the second stage of 13S globulin degradation; the products of metalloproteinase protein activity hydrolyzed into small peptides and amino acids at acid pH (5.6) by cysteine proteinase and carboxypeptidase which appear in germinating seeds [47]. It was clear that cysteine proteinase is able to hydrolyze only the modified l3S globulin but not the native. The role of carboxypeptidase is to facilitate the flow of storage protein hydrolysis and works in cooperation with cysteine proteinase. At latest stage when pH becomes more acidic (5.0) in the vacuoles, aspartic proteinase which is present in dry seeds is involved into the course of hydrolysis protein bodies.
2.2. Hydrolysis of storage seed starch
Carbohydrates represent the most storage food constituent in cereal grains, whereas it contains about 70–80% starch, about 15% protein, less than 5% lipids, minerals and vitamins. In cereals, most hydrolysis enzymes are produced in the aleurone or scutellum in response to germination signals. Several modified seed systems were used to detect the induction process and identify potential factors controlling enzyme induction in absence of the embryo [48].Chrispeels and Varner [49] observed that isolated aleurone failed to synthesize α-amylase in a manner quantitatively similar to distal half seeds led to correction by adding calcium to the medium. The role of calcium might be expected to involve amylase stability, and to have a much more complex involvement in regulating enzyme activities [50]. Because of de novo amylase synthesis during seed germination to stimulate the stored starch mobilization for providing young plant till photosynthesis will be initiate, amylase has been showed high activity [51]. Parys et al. [52] showed that the amylase activity is regulated by the concentration of reducing sugars in vivo in both cotyledons and axis. At the time, the amylase activity in the cotyledons increased gradually and reached a maximum on the 5th day of germination process, while the starch decreased and soluble sugars increased [53].
Many studies which concerned with studying the essentiality of α-amylase activity during seed germination under drought stress and could be summarized as follows; the promotion of drought stressed germinating seeds is a result of high α-amylase activity directly but, it might be related to adaptive strategy to water deficit since its activity is required for solutes accumulation and decrease osmotic potential [54, 55]. In addition, α-amylase synthesis inhibition might be not a mechanism by which drought prevents the germination of Agropyron desertorum seeds [54]. GAs can alleviate the drought stress-caused inhibition of seed germination through regulation of α-amylase [19].
2.3. Hydrolysis of storage seed lipids
Generally oilseeds composed of two parts, the kernel which is main part and the seed covering that enclosed the kernel and called the husk or tegument. The kernel comprised two parts which are the embryo and the endosperm. Lipase activity is investigated during seed germination where it is maximum value [56, 57]. Triacylglycerols is stored in oleosomes and comprise in range from 20 to 50% of dry. As germination proceeds, triacylglycerols are hydrolyzed to produce energy which required for the synthesis of sugars, amino acids (mainly asparagine, aspartate, glutamine and glutamate) and carbon chains required for embryonic growth [58].Lipid level and lipase activity were studied in various germinating seeds. It was showed that β-oxidation takes place 4 days after germination of Castor been seeds [59]. The major hydrolytic enzymes concerned with the lipid metabolism during germination are the lipases which catalyze the hydrolysis of ester carboxylate bonds and releasing fatty acids and organic alcohols [60, 61] and the reverse reaction (esterification) or even various transesterification reactions [62]. The ability of lipases to catalyze these reactions with great efficiency, stability and versatility makes these enzymes highly attractive from a commercial point of view.
Villeneuve [63] and others classified lipases specificities into three main groups; the 1st group is substrate specificity in which glycerol esters represent the natural substrates, the 2nd group is called regioselective and involves the subgroups non-specific lipases that hydrolyze the triacylglycerols into fatty acids and glycerol in a random way with production of mono- and diacylglycerols as intermediate products (Figure 1); specific 1.3 lipases which catalyze the hydrolysis at C1 and C3 glycerol bonds in triacylglycerols with liberating of fatty acids and unstable intermediates 2-monoacylglycerols and 1.2-or 2.3-diacylglycerols and specific or selective type fatty acid that hydrolyze the ester bond of a specific fatty acid or a specific group of fatty acids at any position of triacylglycerol. The 3rd group enantioselective could identify enantiomers in a racemic mixture. The enantio specificities of lipases depend on the type of substrate [64].

Figure 1.
Regioselective: non-specific and 1,3 specific lipases catalyze the hydrolysis of triglycerides in different manners with the production of fatty acids.
The induction of lipase activity during germination might be dependent on factors from embryo [65]. Early study of Shoshi and Reevers [66] showed the presence of two lipases in the endosperm of Castor been seed, acid lipase in dry seed and alkaline lipase during germination. On the other hand, storage tissues of all the oilseeds except Castor bean contained only lipase activity which increased during germination [67].
Because of sucrose is the substrate for lipid biosynthesis in developing seed and the end product of lipid degradation, it might be primarily considered as regulatory factor in studying the mechanisms of lipid metabolism [58, 68]. In addition, asparagine and nitrate are considered regulatory factors in lipid metabolism of lupine [69]. In lupin germinating seeds, the level of asparagine can reach 30% of dry matter, and it is a main transport form of nitrogen from source to sink tissues [70]. Borek et al. [71] reported that asparagine controls the metabolism of carbohydrate as it caused a significant decrease in soluble sugars and increase in starch in organs of germinating lupin seed. In contrast, nitrate is not a favorable source of nitrogen in protein metabolism in lupin seeds [72] and rather does not influence the carbohydrate metabolism [71]. Nitrate similarly as N sucrose, is regarded as a factor which can regulate plant metabolism by changes in the expression of some genes [73].
Storage lipid mobilization in germinating seeds begins with hydrolysis of triacylglycerols in oleosomes by lipases into free fatty acids and glycerol. Then fatty acids undergo β-oxidation in peroxisomes. Next, glyoxylate cycle will proceed partially in the peroxisome and partially in the cytoplasm. Three of the five enzymes of the glyoxylate cycle (citrate synthase, isocitrate lyase and malate synthase) are located in peroxisomes, while two other enzymes (aconitase and malate dehydrogenase) operate in the cytoplasm [74]. Succinate transported from peroxisome to mitochondria and here is converted to malate via the Krebs cycle. Malate in turn, after transport to the cytoplasm, is converted to oxaloacetate. Finally, gluconeogenesis and the synthesis of sugars are the processes which are a form of carbon transport especially in germinating seeds proceed [58, 75].
2.4. Hydrolysis of phytic acid during seed germination
The greatest storage form of total phosphorus (about 50–80%) is phytic acid (C6H18O24P6) and also known as inositol hexophosphate (IP6) in legumes and cereals seeds [76]. Phytic is regarded as antinutrient because it has the ability to form complexes with proteins and bind with cations (especially Fe, Ca, K, Mn, Mg, Zn) via ionic association to form a mixed salt called phytin or phytate with the reduction of their digestive availability [77]. On the other hand, phytate may play an important role as an antioxidant by forming iron complex that cause a decrease in free radical generation and the peroxidation of membranes, and may also act as an anticarcinogen, providing protection against colon cancer [78]. Because of it was regarded as antioxidant, anticarcinogen or vitamin like substance, it is essential to measure and manipulate phytate content in food grains such as beans [79, 80].One of the major breeding objectives is the development of crop cultivars with low seed phytin content. It was found that the increase in myo-inositol and reduced amounts of myo-inositol phosphate intermediates in the seeds of maize mutants with a phenotype of reduced phytic acid had a little effect on plant growth and development [81]. These findings might suggest that a high level of stored phytate is not necessary for seed viability and germination or seedlings growth.
Phytin is mainly stored in protein bodies in seeds called globoids in the aleurone layer and scutellum cells of most grains. Phytic acid has a strong ability to chelate multivalent metal ions, specially zinc, calcium, iron and as with protein residue. Seed phytate content depend mainly on the environmental mainly plant phosphorus fertilization [82]. It has been shown the important genetic variability in the phytate content of beans and it appears to be a trait controlled by several genes [83]. Also, a correlation between phytate and protein contents was found [84], so the protein content of grains can be considered another factor that regulates phytate content.
Phytin in germinating seeds is hydrolyzed by an acid phosphatase enzyme called phytase [85], with releasing of phosphate, cations, and inositol which are utilized by the seedlings. It was found little changes in extractible Pi in hazel seeds during chilling accompanied with IP6 mobilization that might be suggested the rapid conversion of Pi into organic form [86]. These results were discussed as evidence of active metabolism in germinating seed [87]. In agreement, phytase is strongly and competitively inhibited by Pi, while the decrease in phytase activity coincided with maximal IP6 turnover [88]. It was found that about 87% of IP6 is digested during the first 6 days of germination [89]. In this respect, Ogawa et al. [90] postulated that the early axiferous IP6 digestion is essential for metabolic activity of the resting tissue via supplying Pi and minerals for physiological and metabolic requirements, for example, enzymes of starch metabolism. In addition, IP6 related compounds such as pyrophosphate-containing inositol phosphates (PP-IP) play a potential role in providing Pi for ATP synthesis during the early stages of germination before complete dependence on aerobic mitochondrial respiration the mainly source of ATP production [91].
In stressed seeds, many vital processes such as germination, growth, respiration and other related processes are affected which consequently can trigger other effects on metabolic activities particularly the enzymes of phosphate metabolism that play an important role in germination and seed development [92]. Phosphate metabolism is one of negatively affected processes under different stressful conditions [93]. Under stressful conditions, the restriction of growth and phosphorus availability resulting in enhancement the activity of phosphatases to produce Pi by hydrolysis the insoluble phosphate form that modulate mechanism of free phosphate uptake. In agreement, Olmos and Hellin [94] reported that acid phosphatases activity increased to sustain Pi level which enables it to be co-transported with H+ down a proton motive force gradient.
3. Effect of abiotic stress on metabolic activities during seed germination
Abiotic stresses including salt, drought, heavy metals, pollutants, heat, etc., potentially affect seed germination and seedling growth. Depending on the stress intensity and genetic background, germination is delayed or suppressed. Plants have developed unique strategies including a tight regulation of germination ensuring species survival [95]. It was well known that stress exposure would produce early signals such as change in intracellular Ca2+, secondary signaling molecules such as inositol phosphate and ROS as well as activation of kinase cascades.Seed imbibition triggers many biochemical and cellular processes associated with germination involve the reactivation of metabolism, the resumption of cellular respiration and the biogenesis of mitochondria, the translation and/or degradation of stored mRNAs, DNA repair, the transcription and translation of new mRNAs, and the onset of reserve mobilization [7, 96]. These processes are followed by ROS (mostly H2O2) accumulation as a result of a pronounced increase in the intracellular and extracellular production during early stages [97, 98].
ROS function as cellular messengers or toxic molecules on seed hydration [99]. ROS caused seed damage accompanied with a loss of seed vigor and as a repercussion of aging [100]. The highly activity of respiration during germination results in superoxide anion production during electron leakage from the mitochondrial electron transport chain followed by dismutation to H2O2. Other sources of ROS are NADPH oxidases of the plasma membrane, extracellular peroxidases, β-oxidation pathway in glyoxysomes [97]. H2O2 is along-lived ROS that can diffuse easily through membranes and that can reach targets far from production sites, and is recognized as an important signaling molecule [101]. H2O2 is considered as strong oxidizing agent, it could interact with most biomolecules resulting in oxidative stress that causes cellular damage. It causes lipid peroxidation which in turn affects polyunsaturated fatty acids (PUFAs) found in membranes or reserve lipids. Also, H2O2 cause oxidation of nucleic acids (DNA, RNA) and proteins [97]. Induction of DNA oxidation by H2O2 resulted in the accumulation of 7, 8-dihydro-8-oxoguanine (8-oxo-dG), which has been shown to cause the accumulation of double- strand breaks in genome and deleterious effects on cell viability [102]. DNA oxidation by ROS is considered a main source of DNA damage during seed storage and germination.
Kong and Lin [103] have shown that mRNA is much more sensitive to oxidative damage than DNA, mainly due to its cellular localization, single stranded structure and lack of repair mechanisms. Guanine is the most frequently oxidized base in RNA leads to the accumulation of 8-hydroxyguanosine (8-OHG). Oxidative damage to mRNA results in the inhibition of protein synthesis and in protein degradation [104]. Oxidation of protein by ROS result in alteration of protein functions due to enzymatic and binding properties modifications [105]. H2O2 accumulation and associated oxidative damages together with a decline in antioxidant mechanisms can be regarded as a source of stress that may suppress germination. On the other hand, Barba-Espin et al. [106] reported that the selective oxidation of proteins and mRNAs can act as a positive regulator of seed germination.
Using of calcium sensors called Ca2+ binding proteins revealed an increase in intracellular calcium concentration under abiotic-stress conditions [107]. This is accompanied with enhancement of calcium-dependent protein kinases (CDPKs), calcium/calmodulin-dependent protein kinases (CCaMKs) or phosphatases which stimulate the phosphorylation/or dephosphorylation of specific transcription factors, resulting in an increase of stress-responsive genes expression [108]. However, activated Ca2+ sensors regulate stress-responsive genes either by binding to cis-elements in the promoters or by interacting with DNA-binding proteins of genes that led to gene activation or suppression.
Stressed-germinating wheat seeds develop a powerful regulator mechanism in response to stresses which is calreticulin-like protein (M16 and M13) and abundant Ca2+-binding protein predominantly located in the endoplasmic reticulum (ER) of higher plants [109]. Its expression trend was mainly up-regulated, especially in the last period of germination which hints that wheat seed may encounter stress in late germination [110]. Another regulator mechanism with peptidyl-prolyl cis-trans isomerase activity which involved in signal transduction, cell apoptosis, and protein folding called cyclophilin (M51) was detected in stressed germinating wheat seeds [111]. Because of the cellular structure is not complete in early germination, M51 increased slowly in first three germination stages but increased sharply in the last stage [109].
One of the most effective factors on seed imbibition and germination is the temperature. It affects water uptake and reactivation of metabolic processes [7]. Many physiological, biochemical and molecular disturbance will occur with temperature deviation away from optimal to sustain cellular homeostasis [112].
4. The role of phytohormones during germination
Plants are characterized by producing various types of growth regulators that differed in their chemical structure and physiological action. They include auxins, cytokinins (CK), gibberellins (GA), abscisic acid (ABA), ethylene (ET), salicylic acid (SA), jasmonates (JA), brassinosteroids (BR) and strigolactones. Each of ABA, SA, JA and ET is found to play an essential role in mediating plant defense response against stresses [113]. During the early phase of seed germination, a decrease in JA and SA contents and an increased level of auxins were recorded in Arabidopsis seeds [114]. Both JA and SA were shown to act as negative regulators of seed germination [115]. Auxins are considered to be regulators of the seed germination process in a crosstalk with GAs, ABA, and ET [116]. The brassinosteroids signal could stimulate germination by decreasing the sensitivity to ABA [117].A variety of cellular processes in plants are under control of phytohormones which play key roles and coordinate various signal transduction pathways during abiotic-stress response [118]. Seed imbibitions resulted in an activation of GA biosynthesis and response pathways with the production of the bioactive GAs. Then, GAs stimulated the genes encoding for enzymes such as endo-β-1,3 glucanase [119], β-1,4 mannan endohydrolase [120] which hydrolyze the endosperm and alleviate the inhibitory effects of ABA on embryo growth potential [121]. These results are indicated the antagonistic relation between each of ABA and GA which interpret the presence of high GA and low ABA levels in seeds under favorable environmental conditions and a reverse ration under unfavorable conditions. Thus, the crosstalk relation between seed dormancy and germination is balanced by GA-ABA ration, a key mechanism for cope early abiotic-stress conditions.
ABA inhibits water uptake by preventing cell wall loosening of the embryo and thereby reduces embryo growth potential [122]. GAs are involved in direct enhancement the growth of the embryo during late phase [123]. GAs repressive the ABA effect during the early and the late phases of germination through stimulation of genes expression encoding cell wall loosening that result in remodeling enzymes such as α-expansins in early phase of germination. Light and cold act together to break dormancy of imbibed seeds and to promote seed germination by increasing GAs levels. A rapid decrease of ABA endogenous content during Phase II is one of many factors that influence the successful completion of germination [124]. Highly leakage of cellular solutes due to initial imbibition indicates cellular membranes damage caused by rehydration. In addition, drying and rapid seed dehydration processes influence DNA integrity [125]. Seeds have developed a number of repair mechanisms during seed germination, including the repair of membranes, as well as proteins and DNA [126].
Under stress conditions, phytohormones play a crucial role via responsive protein mediated stress. It was found C1-(cysteine rich protein family) domain containing proteins that play a part in plant hormone-mediated stress responses [127]. In addition, 72 responsive proteins mediated stress are identified in Arabidopsis that contained all three unique signature domains. Many hydrolytic enzymes biosynthesis and activity are influenced by GA3 in wheat and barley. Catalase and ascorbate peroxidase activity showed a significant improvement in wheat SA- and GA-primed wheat seeds compared to the unprimed [128, 129].
5. Priming strategy to improve seed germination under stressful or non-stressful conditions
Under various conditions, the potential of seeds for rapid uniform emergence and development under various conditions is determined mostly by seed vigor trait [130]. Recent strategies for improvement seed quality involved classical genetic, molecular biology and invigoration treatments known as priming treatments. Seed priming was aimed primarily to control seed hydration by lowering external water potential, or shortening the hydration period, because of most seeds are partially hydrated after priming process and reach a pre-germinate stage without radicle protrusion [131]. It was reported that primed seeds showed improved germination rate and uniformity under both optimal and adverse environments in wheat [132]. The cellular mechanism of priming as it relates to improved stress tolerance in germinating seeds is still required more study.Currently seed priming techniques include osmopriming (soaking seeds in osmotic solutions as PEG or in salt solutions), hydropriming (soaking seeds in predetermined amounts of distilled water or limiting imbibition periods), and hormone priming (seed are treated with plant growth regulators) which are more commonly studied in laboratory conditions, and thermopriming (it is a physical treatment achieved by pre-sowing of seeds at different temperature that improve germination vigor under adverse environmental conditions) and matric priming (mixing seeds with organic or inorganic solid materials and water in definite proportions and in some cases adding chemical or biological agents) [130, 133]. Hydropriming and osmopriming with large-sized priming molecules cannot permeate cell wall/membrane so water influx would be the only external factor affecting priming. The determination of suitable priming technique is dependent mainly on plant species, seed morphology and physiology. On the other hand, salts and hormone priming affect not only the seed hydration but also other germination-related processes due to absorption of exogenous ions/hormones, consequently confusing the effects of imbibition versus that of ions/hormones.
Improvement germination performance of primed seeds may be considered a result of advanced metabolism processes [134] including enhancement each of the efficiency of respiration [135] and antioxidant activity [136], initiation of repairing processes [137] and alteration phytohormonal balance [138]. Also, improvement of germination performance may be linked to higher expressions of gene sand proteins involved in water transport, cell wall modification, cytoskeletal organization, and cell division and increases in protein synthesis potential, post-translational processing capacity, and targeted proteolysis have been linked to the advanced germination of primed seeds [139].
Seed germination process is regulated by a network of transcription factors that have both confused and separate functions. In order to maintain or break the period of arrested germination and to complete germination under stress conditions, different metabolic pathways including phytohormones biosynthesis and signal transduction pathways, chromatin modifications, and microRNA post transcriptional regulation, are involved [140].
Many effects on metabolic processes, germination performance and seedling establishment due to seed priming with H2O2 were observed although seed soaking followed by dehydration have an important role in controlling gene expression and biosynthesis of proteins [141].
Seed priming with auxin, cytokinin, GA, and ethylene (ET) resulted in improvement of germination of pigeon pea seeds under both control and Cd-stress conditions [142]. ABA pretreated seeds showed a reduction in germination that may be attributed to metabolic deviation, limiting the available energy and changes in metabolomics or may be attributed to modulate the endogenous ABA level [143]. On contrary, GA3 seed treatment has not affect seed germination substantially. It is documented that GA3 have a stimulatory effects on germination and associated enzymes [144]. Also, auxin namely IAA is documented to regulate seed dormancy and plant shade avoidance syndrome that adversely affects seedling development and crop yield [145]. Cytokinin pretreatment may act as auxins in promoting seed germination by antagonizing the inhibitory effect of ABA on germination process. However, it was found that cytokinin antagonize the inhibitory effect of ABA on post-germinating growth of Arabidopsis through the stimulation of ABI5 protein degradation [146].
Recently published data support the existence of interactions between ROS and phytohormone signaling networks that modulate gene expression and cellular redox status [147]. Interaction between phytohormones and H2O2 can be antagonistic or synergistic. Signaling processes trigger interactions are not developed only between particular phytohormones but also between phytohormones and other signaling molecules such as NO [148], H2S [149], ·OH [150] and H2O2 [151], which is believed to play a central role in signaling processes during plant development and stress responses [152]. GA treatment enhanced ROS production namely superoxide and H2O2 in radish plants [153] and Arabidopsis [154]. On the other hand, exogenous application of H2O2 does not influence ABA biosynthesis and signaling but it has a more pronounced effect on GA signaling, resulting in the modulation of hormonal balance and in subsequent germination initiation [154]. It was showed that H2O2 diminished the inhibitory effects of ABA on endosperm damage. Müller et al. [155] showed that H2O2 abolishes inhibitory effects of ABA on endosperm rupture. As suggested previously by Lariguet et al. [154], H2O2 regulates the expression of gene encoding enzyme hydrolyzing the testa and endosperm, which facilitate Arabidopsis germination by releasing the embryo from the control of the seed envelope.
6. The respiratory reactivation during seed germination
The initial liberation of seed stored food at the beginning of germination is mainly by anaerobic respiration. Anaerobic respiration is catalyzed by the activity of enzymes which are not required aerobic conditions such as dehydrogenases [156]. Dehydrogenase facilitating the transport of electrons from substrates to oxygen through electron transport chain using nicotinamide adenine dinucleotide (NAD+), nicotinamide adenine dinucleotide phosphate (NADP+) or riboflavin as cofactor [157]. Activities of dehydrogenases have been shown to involve the activities of alcohol dehydrogenase, lactate dehydrogenase and succinate dehydrogenase [158] which mediated the conversion of storage lipid and carbohydrates through the anaerobic respiration. Succinate dehyrogenase, a complex enzyme tightly bound to the inner mitochondrial membrane oxidizes succinate to fumarate [159]. Lactate dehydrogenase catalyzes the reversible oxidation of lactate to pyruvate using NAD+ as a co-enzyme. Anaerobic respiration was recorded to take place during resting stages of seeds and the initial stages of seed germination [160]. It was showed that the reactivity of dehydrogenases covered the first 3 days of cowpea seed germinations [161].The increase in respiratory rate in germinating seeds is associated with the increase in glycolytic activity. The intermediates of glycolysis are transferred to the OPPP pathway which feeds its products back into glycolysis, so the activity of this pathway is also important in determining the flux through glycolysis [162]. During germination, seeds use sugars and other molecules as a substrate for respiration. α-amylase and β-amylase are involved in degradation of endosperm starch. Starch hydrolysis into glucose is catalyzed by action of α- and β-amylases, debranching enzyme and α-glucosidases (maltase) [163]. So, importance of amylases is related to their ability to provide growing embryo with respiratory substrates for producing energy and carbon source until the established seedling can photosynthesize. In addition, embryo growth from quiescent stage to active phase depending mainly on the utilization of stored ATP and storage lipid breakdown products [164].
Seed germination represents a good period for mitochondria development study. Results obtained from previous transcriptome studies recorded a substantial increase in mitochondrial transcripts encoding proteins and protein content accompanied with changes in their functions during early 3 h of seed imbibitions [165]. During the first 48 h of seed imbibitions, 56 differentially expressed proteins were detected which include the outer membrane channel TOM40 and the inner membrane TIM17/22/23 families, compared to dry seed.
The interpretation of suggestion that import pathway capacity is absolutely dependent on the presence of oxygen (aerobic respiration) is related to the significant decrease in capacity of the general import pathway in mitochondria under anaerobic conditions, compared to under aerobic conditions. In supporting for this suggestion, three proteins from the TIM17/22/23 family were found to be 6–14 folds up-regulated under anaerobic conditions [166] and a decline in proteins involving import apparatus was detected in the mature mitochondria that might be suggested that the accumulation of these import proteins in the dry seed could operate functions after 2 h imbibition, and then serve as donors of TCA cycle and electron transport chain components [167].
7. The role of glyoxylate cycle in oilseed germination
Glyoxylate cycle has been known to play a crucial role in lipid degradation in oilseeds, whereas stored lipid is converted into glucose the main respiratory substrate during germination and hence seedling establishment [168]. Seed imbibition triggers highly increase in oxygen consumption which reflects the enhancement of oxidation of produced carbohydrates from the glyoxylate cycle [169]. Alongside to glyoxylate cycle, the OPPP operates where a number of enzymes and intermediates participate the two pathways [170]. It functions to provide the cell with NADPH for biosynthetic reactions and appears to be important in the regulation of germination [171].The action of the two glyoxylate cycle enzymes isocitrate lyase (ICL) and malate synthase (MS) that by pass the decarboxylation steps of the TCA cycle are essential in oilseed germination. Whereas, two moles of acetyl-CoA are introduced with each turn of the cycle, resulting in the synthesis of one mole of the four-carbon compound succinate that are transported from the glyoxysome into the mitochondrion and converted into malate via TCA cycle. This malate is then exported to cytosol in exchange for succinate and is converted to oxalacetate. PEP-CK catalyzes the conversion of oxaloacetate to phosphoenolpyruvate and this fuels the synthesis of soluble carbohydrates necessary to germination [169].
8. Conclusion
Under stressful conditions, oxidative damage to mRNA results in the inhibition of protein synthesis and in protein degradation which caused disturbance in protein functions due to enzymatic and binding properties modification. Consequently; seed germination may delay or suppress. The priming techniques improve stress acclimation mechanisms during germination but the cellular mechanism of priming is still requires more studying. In response to abiotic stresses, activity of acid phosphatases increased to match a definite level of inorganic phosphate which can be co-transported with H+ down proton motive force gradient. The signaling interactions among multiple phytohormones are rather common in controlling various growth and developmental processes. Hormonal signaling coordination may be regulated through controlling biosynthesis of certain phytohormone, by modifying the available pool of hormone molecules or by elaborate regulation of the signaling process. However; seed pretreatment with each of GAs, auxins or cytokinin promote seed germination not only through stimulation of hydrolyzing enzymes but also by antagonizing the inhibitory effect of ABA on germination process. Phytohormone signal crosstalk will present valuable new avenues for genetic improvement of crop plants needed to meet the future food production targets in the face of global climate change. Surprising; seed priming with H2O2 resulted in improvement germination process and seedling establishment. This may be resulted from its effect on GA signaling and modulation of hormonal balance that promote initiation of seed germination. In addition; H2O2 diminished the inhibitory effects of ABA on endosperm damage through expression of gene encoding enzyme hydrolyzing the testa and endosperm with the releasing of embryo.
References
- 1.Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. International Journal of Molecular Sciences. 2013;14:9643-9684
- 2.Borgheretti F, Ferreira AG. Germinação do básico ao aplicado. Porto Alegre: Artmed; 2000. 222 p
- 3.Zienkiewicz Z, Zienkiewicz AK, Rejon JD, Alche JD, Castro AJ, Rodringuez-Garcia MI. Olive seed protein bodies store degrading enzymes involved in mobilization of oil bodies. Journal of Experimental Botany. 2014; 65: 103-115. DOI: 10.1093/jxb/ert355
- 4.Gonçalves JFC, et al. Aspectos fisiológicos bioquímicos de plantas da Amazônia. Projeto Jacaranda Fase II: Pesquisas Florestais na Amazônia Central. Manaus: INPA; 2003. p. 89-101
- 5.Bewley JD, Black M. Physiology of Development and Germination. 2nd ed. New York: Plenum Press; 1994 445 p
- 6.Bewley JD. Seed germination and dormancy. The Plant Cell. 1997;9:1055-1066
- 7.Bewley JD, Bradford K, Hilhorst H, Nonogaki H. Seeds: Physiology of Development, Germination and Dormancy. 3rd ed. New York: Springer; 2013
- 8.Jacobsen JV, Chandler PM. Gibberellin and abscisic acid in germinating cereals. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Boston, MA: Kluwer; 1995. p. 164-193
- 9.Bethke PC, Schuurink R, Jones RL. Hormonal signaling in cereal aleurone. Journal of Experimental Botany. 1997;48:1337-1356. DOI: 10.1093/jxb/48.7.1337
- 10.Lee SJ, Kang JY, Park HJ, Kim MD, Bae MS, Choi HI, et al. DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiology. 2010;153(2):716-727
- 11.Firenzuoli AM, Vanni P, Ramponi G, Baccari V. Changes in enzyme levels during germination of seeds of Triticum durum. Plant Physiology. 1968;43:260-264. DOI: 10.1104/pp.43.2.260
- 12.Carter LM, Chesson JH. Two USDA researchers develop a moisture seeking attachment for crop seeders that is designed to help grower’s plant seed in soil sufficiently moist for germination. Seed World. 1996;134:14-15
- 13.Munns R. Comparative physiology of salt and water stress. Plant, Cell & Environment. 2002;25:239-250
- 14.Singh J, Sastry EVD, Singh V. Effect of salinity on tomato (Lycopersicon esculentum mill.) during seed germination stage. Physiology and Molecular Biology of Plants. 2012;18:45-50
- 15.Kaur S, Gupta AK, Kaur N. Effect of GA3, kinetin and indole acetic acid on carbohydrate metabolism in chickpea seedlings germinating under water stress. Plant Growth Regulation. 2000;30:61-70
- 16.Zeid IM, Shedeed ZA. Response of alfalfa to putrescine treatment under drought stress. Biologia Plantarum. 2006;50:635-640
- 17.Jacobsen JV, Hanson AD, Chandlor PC. Water stress enhances expression of α-amylase gene in barley leaves. Plant Physiology. 1986;80:350-359
- 18.Todaka D, Matsushima H, Morphashi Y. Water stress enhances α-amylase activity in cucumber cotyledons. Journal of Experimental Botany. 2000;51:739-745
- 19.Velasco R, Salamini F, Bartels D. Dehydration and ABA increase mRNA levels and enzyme activity of cytosolic GAPDH in the resurrection plant Craterostigma plantagineum. Plant Molecular Biology. 1994;26:541-546
- 20.Pandey R, Agarwal RM, Jeevaratnam K, Sharma GL. Osmotic stress-induced alterations in rice (Oryza sativa L.) and recovery on stress release. Plant Growth Regulation. 2004;42:79-87
- 21.Baatour O, Kaddour R, Wannes WA, Lachaâl M, Marzouk B. Salt effects on the growth, mineral nutrition, essential oil yield and composition of marjoram (Origanum majorana). Acta Physiologiae Plantarum. 2010;32:45-51
- 22.Job C, Rajjou L, Lovigny Y, Belghazi M, Job D. Patterns of protein oxidation n Arabidopsis seeds and during germination. Plant Physiology. 2005;138:790-802. DOI: 10.1104/pp.105.062778
- 23.Garg N, Manchanda G. ROS generation in plants: Boon or bane? Plant Biosystems. 2009;143:81-96
- 24.Yacoubi R, Job C, Belghazi M, Job D. Proteomic analysis of the enhancement of seed vigour in osmoprimed alfalfa seeds germinated under salinity stress. Seed Science Research. 2013;23(2):99-110
- 25.Yin H, Chen Q, Yi M. Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regulators. 2008;54:45-54
- 26.Zhang Q, Li JJ, Zhang WJ, Yan SN, Wang R, Zhao JF, Li YJ, Qi ZG, Sun ZX, Zhu ZG. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. The Plant Journal. 2012;72:805-816
- 27.Das AB. Bio prospecting and genetic engineering of mangrove genes to enhance salinity tolerance in crop plants. In: Jain SM, Gupta SD, editors. Biotechnology of Neglected and Underutilized Crops. New York: Springer; 2013. p. 385-456
- 28.Fahad S, Nie L, Chen Y, Wu C, Xiong D, Saud S, Hongyan L, Cui K, Huang L. Crop plant hormones and environmental stress. Sustain Agriculture Reviews. 2015;15:371-400
- 29.Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Current Opinion in Plant Biology. 2002;5:33-36
- 30.Ansari O, Choghazardi HR, Sharif Zadeh F, Nazarli H. Seed reserve utilization and seedling growth of treated seeds of mountain ray (Secale montanum) as affected by drought stress. Cercetări Agronomiceîn Moldova. 2012;2(150):43-48
- 31.Patade VY, Maya K, Zakwan A. Seed priming mediated germination improvement and tolerance to subsequent exposure to cold and salt stress in capsicum. Research Journal of Seed Science. 2011;4(3):125-136
- 32.Mayer AM, Shain Y. Control of seed germination. Annual Review of Plant Physiology. 1974;25:167-193
- 33.Bewley JD, Black M. Seeds: Physiology of Development and Germination. New York: Plenum Press; 1985
- 34.Mayer AM, Poljakoff-Mayber A. The Germination of Seeds. 4th ed. Oxford: Pergamon Press; 1989
- 35.Salisbury FB, Ross CW. Plant Physiology. 4th ed. Belmont, California, USA: Wadsworth Publishing Company; 1991
- 36.Shutov AD, Vaintraub IA. Degradation of storage proteins in germinating seeds. Phytochemistry. 1987;26:1557-1566
- 37.Gepstin S, Han I. Evidence for the involvement of cytokinin in the regulation of proteolytic activity in cotyledons of germinating beans. Plant and Cell Physiology. 1980;21(1):57-63
- 38.Shivaraj B, Pattabiraman TN. Natural plant enzyme inhibitors, part VIII. Indian Journal of Biochemistry & Biophysics. 1980;17:181-185
- 39.Veerabhadrappa PS, Manjunath NH, Virupaksha TK. Proteinase inhibitors of finger millet (Eleusine coracana gaertn.). Journal of the Science of Food and Agriculture. 1978;29(4):353-358
- 40.Tully RE, Beevers H. Protease and peptidases of castor bean endosperm. Enzyme characterization and changes during germination. Plant Physiology. 1978;62:726-750
- 41.Schlereth A, Becker C, Horstmann C, Tiedemann J, Müntz K. Comparison of globulin mobilization and cysteine proteinases in embryonic axes and cotyledons during germination and seedling growth of vetch (Vicia sativa L.). Journal of Experimental Botany. 2000;51:1423-1433
- 42.Yano H, Wong JH, Lee YM, Cho MJ, Buchanan BB. A strategy for the identification of proteins targeted by thioredoxin. Proceedings of the National Academy of Sciences USA. 2001;98:4794-4799. DOI: 10.1073/pnas.071041998
- 43.Yano H, Masaharu KM. Disulfide proteome yields a detailed understanding of redox regulations: A model study of thioredoxin-linked reactions in seed germination. Proteomics. 2006;6:294-300. DOI: 10.1002/pmic.200402033
- 44.Dunaevsky YE, Belozersky MA. Proteolysis of the main storage protein of buckwheat seeds at the early stage of germination. Physiologia Plantarum. 1989;75:424-428
- 45.Belozersky MA, Dunaevsky YE, Voskoboynikova NE. Isolation and properties of a metalloproteinase from buckwheat seeds. The Biochemical Journal. 1990;272:677-682
- 46.Elpidina EN, Dunaevsky YE, Belozersky MA. Protein bodies from buckwheat seed cotyledons: Isolation and characteristics. Journal of Experimental Botany. 1990;41:969-977
- 47.Dunaevsky YE, Belozersky MA. The role of cysteine proteinase and carboxypeptidase in the breakdown of storage proteins in buckwheat seeds. Planta. 1989;179:316-322
- 48.Paleg LG. Physiological effects of gibberellic acid II. On starch hydrolysis enzymes of barley endosperm. Plant Physiology. 1960;35:902-906
- 49.Chrispeels MJ, Varner JE. Gibberellic acid enhanced synthesis and release of α-amylase and ribonuclease by isolated barley aleurone layers. Plant Physiology. 1967;42:398-406
- 50.Jones RL, Jacobsen JV. Regulation of synthesis and transport of secreted proteins in cereal aleurone. International Review of Cytology. 1991;126:49-88
- 51.Filner P, Varner JE. A simple and unequivocal test for de novo synthesis of enzymes: Density labeling of barley α-amylase with H2O18. Proceedings of the National Academy of Sciences USA. 1967;58:1520-1526
- 52.Parys E, Romanowska E, Poskuta J. Amylase activities in attached and excised cotyledons and in embryonic axes of Pisum sativum L. Plant and Cell Physiology. 1983;21:181-188
- 53.Wang Y, Wang DM, Liang HG. Effect on plumular axis on the amylase activity in cotyledons of germinating pea seeds. Acta Phytophysiology Sinica. 1988;14(3):244-249
- 54.Kępczyński J. Ethylene-dependent action of gibberellins in seed germination of Amaranthus caudatus. Physiologia Plantarum. 1986;67:584-587
- 55.Wilson AM. Amylase synthesis and stability in crested wheatgrass seeds at low water potentials. Plant Physiology. 1971;48:541-546
- 56.Hellyer SA, Chandler IC, Bosley JA. Can the fatty acid selectivity of plant lipases be predicted from the composition of the seed triglyceride? Biochemica Biophysica Acta. 1999;1440(2-3):215
- 57.Paques FW, Macedo GA. Lipases de Látex Vegetais: Propriedades e Aplicações Industriais: A Review. Química Nova. 2006;29(1):93
- 58.Quettier AL, Eastmond PJ. Storage oil hydrolysis during early seedling growth. Plant Physiology and Biochemistry. 2009;47:485
- 59.Hutton D, Stumpf PK. Fat metabolism in higher plant. Characterisation of β-oxidation system from maturing and germinating caster bean seeds. Plant Physiology. 1969;44:508-516
- 60.Pereira EP, Zanin GM, Castro HF. Immobilization and catalytic properties of lipase on chitosan for hydrolysis and etherification reactions. Brazilian Journal of Chemical Engineering. 2003;20(4):343
- 61.Leal MCM, Cammarota MC, Freire DMG, Sant’Anna JGL. Hydrolytic enzymes as coadjuvants in the anaerobic treatment of dairy waste waters. Brazilian Journal of Chemical Engineering. 2002;19(2):175
- 62.Freire GDM, Castilho FL. Lipases em Biocatálise. In: Bon et al. (org), editor. Enzimas em biotecnologia: Produção, Aplicação e Mercado. Rio de Janeiro: Interciência; 2008
- 63.Villeneuve P. Plant lipases and their applications in oils and fats modification. European Journal of Lipid Science and Technology. 2003;105(6):308
- 64.Castro HF, Anderson WA. Fine chemicals by biotransformation using lipases. Quimica Nova. 1995;18(6):544-554
- 65.Tavener RJA, Laidman DL. The induction of triglyceride metabolism in the germinating wheat grain. Phytochemistry. 1972;11:981-987
- 66.Shoshii M, Reevers H. Lipase activity in castor been endosperm during germination. Plant Physiology. 1974;54:23-28
- 67.Anthony HC, Huang AHC, Moreau RA. Lipases in the storage tissues of peanut and other oil seeds during germination. Planta. 1978;141:111-116
- 68.Baud S, Dubreucq B, Miquel M, Rochat C, Lepiniec L. Storage reserve accumulation in Arabidopsis: Metabolic and developmental control of seed filling. Arabidopsis Book American Society of Plant Biology. 2008;6: e0113. DOI: 10.1199/tab.0113
- 69.Ratajczak W. Asparagine metabolism in developing seeds of Lupinus luteus L. Biochemie und Physiologie der Pflanzen. 1986;181:17-22
- 70.Lehmann T, Ratajczak L. The pivotal role of glutamate dehydrogenase (GDH) in the mobilization of N and C from storage material to asparagine in germinating seeds of yellow lupine. Journal of Plant Physiology. 2008;165:149-158. DOI: 10.1016/j.jplph.2006.12.010
- 71.Borek S, Galor A, Paluch E. Asparagine enhances starch accumulation in developing and germinating lupin seeds. Journal of Plant Growth Regulation. 2013;32:471-482
- 72.Ratajczak W, Gwóźdz´ EA, Mia˛dowicz M. Effects of nitrogen nutrition on storage protein composition yellow lupin cotyledons cultured in vitro. Acta Physiologiae Plantarum. 1996;18:295-304
- 73.Vidal EA, Gutiérrez RA. A systems view of nitrogen nutrient and metabolite responses in Arabidopsis. Current Opinion in Plant Biology. 2008;11:521-529
- 74.Pracharoenwattana I, Smith SM. When is a peroxisome not a peroxisome? Trends in Plant Science. 2008;13:522-525
- 75.Borek S, Ratajczak L. Storage lipids as a source of carbon skeletons for asparagine synthesis in germinating seeds of yellow lupine (Lupinus luteus L.). Journal of Plant Physiology. 2010;167:717-724
- 76.Jacela JY, DeRouehey Tokach MD, Goodband RD, Nelssen JL, Renter D, Dritz SS. Feed additives for swine: Fact sheets-prebiotics and probiotis and phytogenics. Journal of Swine Health and Production. 2010;18:87-91
- 77.Lott JNA, Greenwood JS, Batten GD. Mechanisms and regulation of mineral nutrient storage during seed development. In: Kigel J, Galili G, editors. Seed Development and Germination. New York: Marcel Dekker Inc; 1995. p. 215-235
- 78.Thompson LU, Zhang L. Phytic acid and minerals: Effect on early markers of risk for mammary and colon carcinogenesis. Carcinogenesis. 1991;12:2041-2045
- 79.Coelho SM, Taylor AR, Ryan KP, Sousa-Pinto I, Brown MT, Brownlee C. Spatiotemporal patterning of reactive oxygen production and Ca2+ wave propagation in Fucus rhizoid cells. The Plant Cell. 2002;14:2369-2381
- 80.Okazaki Y, Katayama T. Reassessment of the nutritional function of phytic acid, with special reference to myo-inositol function. Journal of Japan Society of Nutrition and Food Sciences. 2005;58:151-156
- 81.Shi H, Bressan R, Hasegawa PM, Zhu J-K. In: Broadlay M, White P, editors. Sodium in Plant Nutritional Genomics. London: Blackwell Publishing; 2005. p. 127-149
- 82.Buerkert A, Haake C, Ruckwied M, Marschner H. Phosphorus application affects the nutritional quality of millet grain in the sahel. Field Crops Research. 1998;57:223-235
- 83.Santos JCP. Estado nutricional do feijoeiro (Phaseolus vulgaris L.) e teores de nutrientes e fitatos nos grãos. Piracicaba: Universidade de São Paulo. Tese de doutorado; 1998
- 84.Raboy V, Noaman MM, Taylor GA, Pickett SG. Grain phytic acid and protein are highly correlated in winter wheat. Crop Science. 1991;31:631-635
- 85.Hubel F, Beck E. Maize root phytase. Purification, characterization, and localization of enzyme activity and its putative substrate. Plant Physiology. 1996;112:1429-1436
- 86.Mukherji S, Dey B, Paul AK, Sircar SM. Changes in phosphorus fractions and phytase activity of rice seeds during germination. Physiologia Plantarum. 1971;25:94-97
- 87.Silva LG, Trugo LC. Characterization of phytase activity in lupin seed. Journal of Food Biochemistry. 1996;20:329-340
- 88.Andriotis VME, Ross JD. Isolation and characterization of phytase from dormant Corylus avellana seeds. Phytochemistry. 2003;64:689-699
- 89.Azarkovich MI, Dmitrieva MI, Sobolev AM. Mobilization of protein and phytin in aleurone grains of germinating castor beans. Russian Journal of Plant Physiology. 1999;46:349-356
- 90.Ogawa M, Tanaka K, Kasai Z. Accumulation of phosphorus, magnesium, and potassium in developing rice grains: Followed by electron microprobe X-ray analysis focusing on the aleurone layer. Plant and Cell Physiology. 1979;20:19-27
- 91.Raboy V. Myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry. 2003;64:1033-1043
- 92.Fincher GB. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annual Review of Plant Physiology. 1989;40:305-346
- 93.Mihoub A, Chaoui A, El-Ferjani E. Biochemical changes associated with cadmium and copper stress in germinating pea seeds (Pisum sativum L.). Competes Rendus Biologies. 2005;328:33-41
- 94.Olmos E, Hellin E. Cytochemical localization of ATpase plasma membrane and acid phosphatase by cerium based in a salt-adapted cell line of Pisum sativum. Journal of Experimental Botany. 1997;48:1529-1535
- 95.Bai B, Sikron S, Gendler T, Kazachkova Y, Barak S, Grafi G, Khozin-Goldberg I, Fait A. Ecotypic variability in the metabolic response of seeds to diurnal hydration–dehydration cycles and its relationship to seed vigor. Plant and Cell Physiology. 2011;53(1):38-52
- 96.Nonogaki H, Bassel GW, Bewley JD. Germination–stillamystery. Plant Science. 2010;179:574-581. DOI: 10.4161/psb.25504
- 97.El-Maarouf-Bouteau H, Bailly C. Oxidative signaling in seed germination and dormancy. Plant Signaling & Behavior. 2008;3:175-182. DOI: 10.4161/psb.3.3.5539
- 98.Kubala S, Wojtyla Ł, Quinet M, Lechowska K, Lutts S, Garnczarska M. Enhanced expression of the proline synthesis gene P5CSA in relations to seed osmopriming improvement of Brassica napus germination under salinity stress. Journal of Plant Physiology. 2015;183:1-12. DOI: 10.1016/j.jplph.2015.04.009
- 99.Bailly C, El-Maarouf-Bouteau H, Corbineau F. From intra cellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biologies. 2008;331:806-814. DOI: 10.1016/j.crvi.2008.07.022
- 100.Kumar SPJ, Prasad SR, Banerjee R, Thammineni C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Annals of Botany. 2015;116:663-668. DOI: 10.1093/aob/mcv098
- 101.Møller IM, Jensen PE, Hansson A. Oxidative modifications to cellular components in plants. Annual Review of Plant Biology. 2007;58:459-481. DOI: 10.1146/annurev.arplant.58.032806.103946
- 102.Pommier Y, Redon C, Rao VA, Seiler JA, Sordet O, Takemura H, et al. Repair of and checkpoint response to topoisomerase I-mediated DNA damage. Mutation Research. 2003;532:173-203. DOI: 10.1016/j.mrfmmm.08.016
- 103.Kong QM, Lin CG. Oxidative damage to RNA: Mechanisms, consequences and diseases. Cellular and Molecular Life Sciences. 2010;67:1817-1829. DOI: 10.1007/s00018-010-0277-y
- 104.Chmielowska-Bkak J, Izbiańska K, Deckert J. Products of lipid, protein and mRNA oxidation as signals and regulators of gene expression. Frontiers in Plant Science. 2015;6:405. DOI: 10.3389/fpls.2015.00405
- 105.Davies MJ. The oxidative environment and protein damage. Biochimica et Biophysica Acta. 2005;1703:93-109. DOI: 10.1016/j.bbapap.2004.08.007
- 106.Barba-Espin G et al. Role of thioproline on seed germination: Interaction ROS-ABA and effects on antioxidative metabolism. Plant Physiology and Biochemistry. 2011;59:30-36. DOI: 10.1016/j.plaphy.2011.12.002.3
- 107.Kudla J, Batistic O, Hashimoto K. Calcium signals: The lead currency of plant information processing. The Plant Cell. 2010;22:541-563
- 108.Reddy AS, Ali GS, Celesnik H, Day IS. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. The Plant Cell. 2011;23(6):2010-2032
- 109.Finnie C, Melchior S, Roepstorff P, Svensson B. Proteome analysis of grain filling and seed maturation in barley. Plant Physiology. 2002;129:1308-1319
- 110.Komatsu S, Yang G. Over-expression of calcium-dependent protein kinase 13 and calreticulin interacting protein 1 confers cold tolerance on rice plants. Molecular Genetics and Genomics. 2007;277:713-723
- 111.Galat A. Variations of sequences and amino acid composition of proteins that sustain their biological function: An analysis of the cyclophilin family of proteins. Archives of Biochemistry and Biophysics. 1999;371(2):149-162
- 112.Fitter AH, Hay RKM. Environmental Physiology of Plants. New York: Academic Press; 1981
- 113.Wani SH, Kumar V, Shriram V, Kumar S. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. The Crop Journal. 2016;4:162-176
- 114.Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, et al. Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: A comparative study on dormant and non dormant accessions. Plant and Cell Physiology. 2009;50:1786-1800
- 115.Dave A, ML H’n, He Z, Andriotis VM, Vaistij FE, Larson TR, et al. 12-Oxo-phytodienoic acid accumulation during seed development represses seed germination in Arabidopsis. The Plant Cell. 2011;23:583-599
- 116.Carrera E, Holman T, Medhurst A, Dietrich D, Footitt S, Theodoulou FL, et al. Seed after-ripening is a discrete developmental pathway associated with specific gene networks in Arabidopsis. The Plant Journal. 2008;53:214-224
- 117.Steber CM, McCourt P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiology. 2001;125:763-769
- 118.Vob U, Bishopp A, Farcot A, Bennett MJ. Modelling hormonal response and development. Trends in Plant Science. 2014;19:311-319
- 119.Leubner-Metzger G, Fründt C, Vögeli-Lange R, Meins FJ. β-1, 3-glucanases in the endosperm of tobacco during germination. Plant Physiology. 1995;109:751-759
- 120.Nonogaki H, Gee OH, Bradford KJ. A germination-specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiology. 2000;123:1235-1245
- 121.Koornneef M, Karssen CM. Seed dormancy and germination. In: Arabidopsis Book/Cold Spring Harbor Monograph Archive. 1994;27:313-334
- 122.Schopfer P, Plachy C. Control of seed germination by abscisic acid. Plant Physiology. 1985;77(3):676-686
- 123.Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiology. 2000;122:415-424
- 124.Weitbrecht K, Müller K, Leubner-Metzger G. First off the mark: Early seed germination. Journal of Experimental Botany. 2011;62:3289-3309
- 125.Matilla A, Gallardo M, Puga-Hermida MI. Structural, physiological and molecular aspects of heterogeneity in seeds: A review. Seed Science Research. 2005;15:63-76
- 126.Oge L, Bourdais G, Bove J, Collet B, Godin B, Granier F, et al. Protein repair-L isoaspartyl methyltransferase 1 is involved in both seed longevity and germination vigor in Arabidopsis. The Plant Cell. 2008;20:3022-3037
- 127.Bhaskar RV, Mohanty B, Verma V, Wijaya E, Kumar PP. A hormone-responsive C1-domain-containing protein At5g17960 mediates stress response in Arabidopsis thaliana. PLoS. 2015;10(1):115-118. DOI: 10.1371/journal.pone.0115418
- 128.Hammerton RW, Ho T-HD. Hormonal regulation of the development of protease and carboxypeptidase activities in barley aleurone layers. Plant Physiology. 1986;80:692-697
- 129.Stuart IM, Loi L, Fincher GB. Development of (1-3, 1-4)-fl-glucan endohydrolase isoenzymes in isolated scutella and aleurone layers of barley (Hordeum vulgare). Plant Physiology. 1986;80:310-314
- 130.Paparella S, Araújo SS, Rossi G, Wijayasinghe M, Carbonera D, Balestrazzi A. Seed priming: State of the art and new perspectives. Plant Cell Reports. 2015;34:1281-1293. DOI: 10.1007/s00299-015-1784-y
- 131.Hilhorst HWM, Finch-Savage WE, Buitink J, Bolingue W, Leubner-Metzger G. Dormancy in plant seeds. In: Lubzens E, Cerdà J, Clarck M, editors. Dormancy and Resistance in Harsh Environments. Heideberg: Springer-Verlag; 2010. p. 43-67
- 132.Zhuo J, Wang W, Lu Y, Sen W, Wang X. Osmopriming-regulated changes of plasma membrane composition and function were inhibited by phenylarsin oxide in soybean seeds. Journal of Integrative Plant Biology. 2009;9:858-867
- 133.Jisha KC, Vijayakumari KJT, Puthur JT. Seed priming for abiotic stress tolerance: An overview. Acta Physiologiae Plantarum. 2013;3:1381-1396. DOI: 10.1007/s11738-012-1186-5
- 134.Soeda Y, Konings MCJM, Vorst O, van Houwelingen AMML, Stoopen GM, Maliepaard CA, Koddle J, Bino RJ, Groot SPC, van der Geest AHM. Gene expression programs during Brassica oleracea seed maturation, osmopriming, and germination are indicators of progression of the germination process and the stress tolerance level. Plant Physiology 2005; 137:354-368
- 135.Sun J, Hutchins DA, Feng Y, Seubert EL, Caron DA, Fu F-X. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnology and Oceanography. 2011;56:829-840. DOI: 10.4319/lo.2011.56.3.0829
- 136.Chen K, Arora R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in spinach (Spinacia oleracea). Plant Science. 2011;180:212-220. DOI: 10.1016/j.plantsci.2010.08.007
- 137.Balestrazzi A, Macovei C, Carbonera D. Seed imbibition in Medicago truncatula Gaertn. Expression profiles of DNA repair genes in relation to PEG-mediated stress. Journal of Plant Physiology. 2011;168:706-713. DOI: 10.1016/j.jplph.2010.10.008
- 138.El-Araby MM, Moustafa SMA, Ismail AI, Hegazi AZA. Hormone and phenol levels during germination and osmopriming of tomato seeds, and associated variations in protein patterns and anatomical seed features. Acta Agronomica Hungarica. 2006;54:441-458. DOI: 10.1556/AAgr.54.2006.4.7
- 139.Kubala S, Garnczarska M, Wojtyla Ł, Clippe A, Kosmala A, Zmiénko A, et al. Deciphering priming-induced improvement of rape seed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Science. 2015;231:94-113. DOI: 10.1016/j.plantsci.2014.11.008
- 140.Hilhorst HWM. Definition and hypotheses of seed dormancy. In: Bradford KJ, Nonogaki H, editors. Seed Development, Dormancy and Germination. Annual Plant Reviews. Vol. 27, Chap 4. Sheffield, UK: Blackwell Publishing; 2007. p. 50-71
- 141.Sneideris LC, Gavassi MA, Campos ML, D’Amico-Damião V, Carvalho RF. Effects of hormonal priming on seed germination of pigeon pea under cadmium stress. Anais da Academia Brasileira de Ciências. 2015;87:1847-1852. DOI: 10.1590/0001-3765201520140332
- 142.Sneideris AC, Gavassi MA, Campiao VDA, Carvalho RF. Effects of hormonal priming on seed germination of pigeon pea under cadmium stress. Annals of the Brazilian Academy of Sciences. 2015;87(3):1847-1852. DOI: 10.1590/0001-3765201520140332
- 143.Fincher GB. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annual Review of Plant Physiology and Plant Molecular Biology. 1989;40:305-346
- 144.Liu W-Z, Kong D-D, Gu X-X, Gao HB, Wang J-Z, Xia M, et al. Cytokinins can act as suppressors of nitric oxide in Arabidopsis. Proceedings of the National Academy of Sciences USA. 2013;110:1548-1553. DOI: 10.1073/pnas.1213235110
- 145.Procko C, Crenshaw CM, Ljung K, Noel JP, Chory J. Cotyledon generated auxin is required for shade-induced hypocotyl growth in Brassica rapa. Plant Physiology. 2014;165:1285-1301. DOI: 10.1104/pp.114.241844
- 146.Guan C, Wang X, Feng J, Hong S, Liang Y, Ren B, et al. Cytokinin antagonizes abscisic acid-mediated inhibition of cotyledon greening by promoting the degradation of abscisic acid insensitive 5 protein in Arabidopsis. Plant Physiology. 2014;164:1515-1526. DOI: 10.1104/pp.113.234740
- 147.Xia X-J, Zhou Y-H, Shi K, Zhou J, Foyer CH, Hu J-Q. Inter play between reactive oxygen species and hormones in the control of plant development and stress tolerance. Journal of Experimental Botany. 2015;66:2839-2856. DOI: 10.1093/jxb/erv089
- 148.Sanz L, Albertos P, Mateos I, Sánchez-Vicente I, Lechón T, Fernández Marcos M, Lorenzo O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. Journal of Experimental Botany. 2015;66:2857-2868
- 149.Jin Z, Pei Y. Physiological implications of hydrogen sulfide in plants: Pleasant exploration behind its unpleasant odour. Oxidative Medicine and Cellular Longevity. 2015;397502. DOI: 10.1155/2015/397502
- 150.Richards SL, Wilkins KA, Swarbreck SM, Anderson AA, Habib N, Smith AG, et al. The hydroxyl radical in plants: From seed to seed. Journal of Experimental Botany. 2015;66:37-46. DOI: 10.1093/jxb/eru 398
- 151.Diaz-Vivancos P, Barba-Espín G, Hernández JA. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Reports. 2013;32:1491-1502. DOI: 10.1007/s00299-013-1473-7
- 152.Petrov VD, Van Breusegem F. Hydrogen peroxide–a central hub for information flow in plant cells. AoB Plants. 2012;2012
ls014. DOI: 10.1093/aobpla/pls014
- 153.Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiology. 2001;125:1591-1602. DOI: 10.1104/pp.125.4.1591
- 154.Lariguet P, Ranocha P, DeMeyer M, Barbier O, Penel C, Dunand C. Identification of a hydrogen peroxide signaling pathway in the control of light-dependent germination in Arabidopsis. Planta. 2013;238:381-395. DOI: 10.1007/s00425-013-1901-5
- 155.Müller K, Hess B, Leubner-Metzger G. A role for reactive oxygen species in endosperm weakening. In: Adkins S, Shmore SA, Navie S, editors. Seeds: Biology, Development and Ecology. Wallingford: CAB International; 2007. p. 287-295
- 156.Turner JF, Turner DH. The regulation of carbohydrate metabolism. Annual Review of Plant Physiology. 1975;26:159-186
- 157.Robert KM, David AB, Katheleen MB, Peter JK, Victor WR, Weil P. A Harper’s illustrated biochemistry. Biologic Oxidation. 2009;12:99-100
- 158.Oaikhena EE, Ajibade GA, Appah J, Bello M. Dehydrogenase enzyme activities in germinating cowpea (Vigna unguiculata (L) Walp). Journal of Biology, Agriculture and Healthcare. 2013;3:32-36
- 159.Devlin T. Text book of biochemistry with clinical correlations. Bioenergetics mitochondria and oxidative. Metabolism. 2011;14:554-555
- 160.Jones JD, Burneth P, Zollman P. The glyoxylate cycle does it function in the dormant or active seed. Comparative Biochemistry and Physiology Part B: Biochemistrty and Molecular Biology. 1999;124(2):177-179
- 161.Ebukanson GJ, Bassey ME. About Seed Plants. Kaduna: Baraka Press and Publishers Ltd; 1992. p. 36-39
- 162.Podestá FE, Plaxton WC. Regulation of cytosolic carbon metabolism in germinating Ricinus communis cotyledons. II. Properties of phosphoenolpyruvate carboxylase and cytosolic pyruvate kinase associated with the regulation of glycolysis and nitrogen assimilation. Planta. 1994;194:381-387
- 163.Andriotis VME, Saalbach G, Waugh R, Field RA, Smith AM. Maltase involved in starch metabolism in barley endosperm is encoded by a single gene. PLoS One. 2016;11(3):e0151642. DOI: 10.1371/journal.pone.0151642
- 164.Nandi S, Das G, Sen-Mendi S. β-amylase activity as an index for germination potential in rice. Annals of Botany. 1995;75:463-467
- 165.Howell KA, Narsair R, Carroll A, Ivanova A, Lohse M, Usadel B, et al. Mapping metabolic and transcript temporal switches during germination in rice high- lights specific transcription factors and the role of RNA in stability in the germination process. Plant Physiology. 2009;149:961-980. DOI: 10.1104/pp.108.129874
- 166.Howell KA, Cheng K, Murcha MW, Jenkin LE, Millar AH, Whelan J. Oxygen initiation of respiration and mitochondrial biogenesis in rice. The Journal of Biological Chemistry. 2007;282:15619-15619
- 167.Howell KA, Millar AH, Whelan J. Ordered assembly of mitochondria during rice germination begins with promitochondrial structures rich in component of the protein import apparatus. Plant Molecular Biology. 2006;60:201-223
- 168.Eastmond PJ, Germain V, Lange PR, Bryce JH, Smith SM, Graham IA. Post germinative growth and lipid catabolism in oilseeds lacking the gloxylate cycle. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5669-5674
- 169.Muscolo A, Sidari M, Mallamaci C, Attinà E. Changes in germination and glyoxylate and respiratory enzymes of Pinus pinea seeds under various abiotic stresses. Journal of Plant Interactions. 2007;2(4):273-279. DOI: 10.1080/17429140701713795
- 170.ap Rees T. Integration of pathways of synthesis and degradation of hexose phosphates. In: Preiss J, editor. The Biochemistry of Plants. London: Academic Press. 1980; pp. 1-42.
- 171.Perino C, Come D. Physiological and metabolic study of the germination phases in apple embryo. Seed Science and Technology. 1991;19:1-14
Last edited:
acespicoli
Well-known member
Seed senescence
Seed germination performance is a major determinant of crop yield. Deterioration of seed quality with age is associated with accumulation of DNA damage.[6] In dry, aging rye seeds, DNA damages occur with loss of viability of embryos.[7] Dry seeds of Vicia faba accumulate DNA damage with time in storage, and undergo DNA repair upon germination.[8] In Arabidopsis, a DNA ligase is employed in repair of DNA single- and double-strand breaks during seed germination and this ligase is an important determinant of seed longevity.[9] In eukaryotes, the cellular repair response to DNA damage is orchestrated, in part, by the DNA damage checkpoint kinase ATM. ATM has a major role in controlling germination of aged seeds by integrating progression through germination with the repair response to DNA damages accumulated during the dry quiescent state.[10]Koppen G, Verschaeve L. The alkaline single-cell gel electrophoresis/comet assay: a way to study DNA repair in radicle cells of germinating Vicia faba. Folia Biol (Praha). 2001;47(2):50-4. PMID: 11321247.


