St. Phatty
Active member
This is one of those Fast & Furious type questions.
Plus, I watched Maloof Racing on Netflix.
They use Nitrous Oxide - for Racing AND for Dental Anaesthesia ?
Now that is one Versatile Chemical Compound ! !!
"The purpose of a nitrous oxide system is **to increase the power output of an engine** . It does this by increasing the amount of fuel that can be burned, by increasing the oxygen supply."
So, if they want more Oxygen - why not use a tank of Compressed Oxygen, and fold that into the inflow of Atmospheric Air, 80% Nitrogen 20% Oxygen 420 ppm CO2 ?
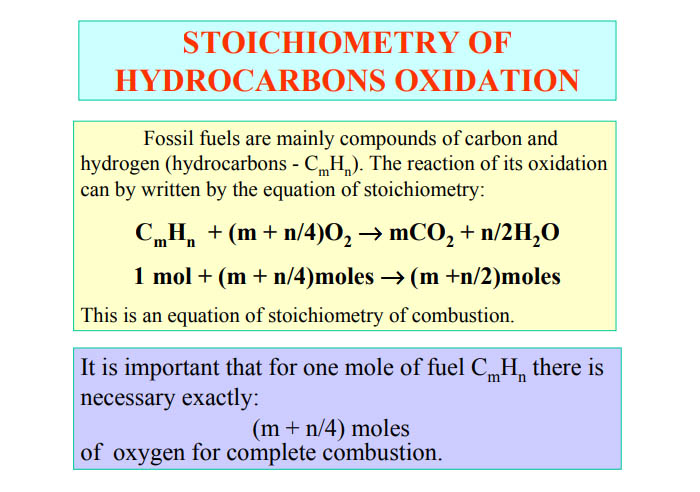
Does anyone know the formula/ Stoichiometry for burning Gasoline with Nitrous Oxide ?
Plus, I watched Maloof Racing on Netflix.
They use Nitrous Oxide - for Racing AND for Dental Anaesthesia ?
Now that is one Versatile Chemical Compound ! !!
"The purpose of a nitrous oxide system is **to increase the power output of an engine** . It does this by increasing the amount of fuel that can be burned, by increasing the oxygen supply."
So, if they want more Oxygen - why not use a tank of Compressed Oxygen, and fold that into the inflow of Atmospheric Air, 80% Nitrogen 20% Oxygen 420 ppm CO2 ?
Does anyone know the formula/ Stoichiometry for burning Gasoline with Nitrous Oxide ?





