So being like a kid in a candy store here at a site filled with so many people with a wealth of knowledge, my mind feels free to explore the how and why of enjoying this gift. Think going from doobs or even home-made pipes from beer cans to Volcano, rosin press and blasting. And meanwhile I am trying to keep the lungs working OK.
So it seems that what is going on as we heat (flower, bubble, rosin, oil, etc) the material the compound react to the temperature and ambient pressure. The "lighter" compounds go at a lower temp and the "heavier" compounds at a higher temp. We can assume the pressure is constant in whatever the material is being heated in.
Following from here: https://chem.libretexts.org/Bookshe...nd_Intermolecular_Forces/11.5:_Vapor_Pressure
If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. Instead, they will diffuse through the gas phase away from the container, and an equilibrium will never be established. Under these conditions, the liquid will continue to evaporate until it has “disappeared.” The speed with which this occurs depends on the vapor pressure of the liquid and the temperature. Volatile liquids have relatively high vapor pressures and tend to evaporate readily; nonvolatile liquids have low vapor pressures and evaporate more slowly.
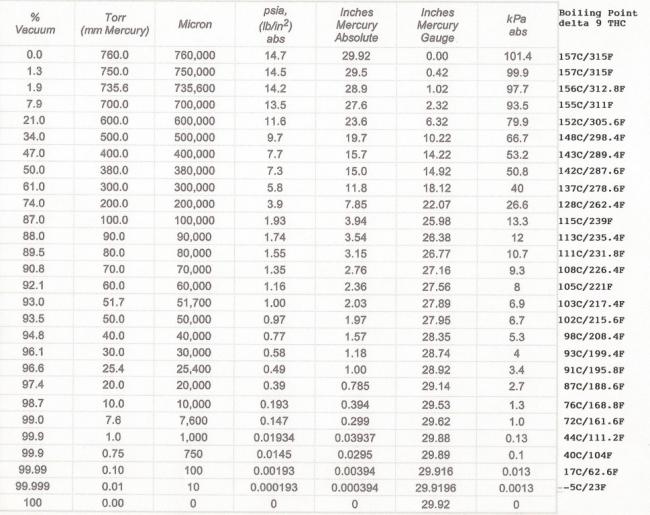
So looking at that chart and imagining it has all of the compounds we care about (some probably lump together) we can see that individual items have vapor "smell" even below the boiling point (vapor pressure is same as local atmosphere). That is why a bag can be sniffed for "quality" - the lower boiling point items are making vapor. Leave the bag sitting and they may all go away leaving thicker vapors to make smell.
I would love to see vapor pressure charts for the desired compounds and be able to see what I get at 300, 360, 420. At 360 there is a lot left behind and that goes into edibles. 420, burned out I think. I also suspect that folks that are heating their material at a high temp to get max vapor are also reaching out to the thicker compounds.
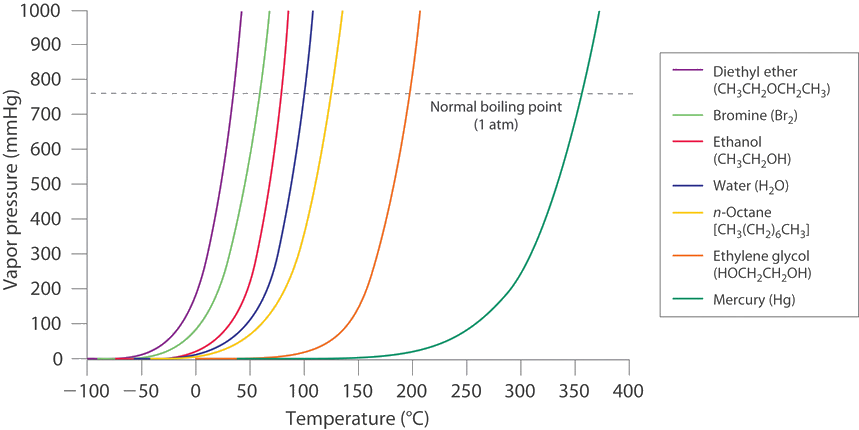
If you look at the log pressure / temp chart on this link, and imagine vaping at 20*, you would get all the lower things in poportion to their individual vapor pressures, and miss entirely the upper two. The difference in strins is the profile of these lines I think.
https://en.wikipedia.org/wiki/Vapor_pressure
IMO this needs some discussion. There is much on the web that dances around this as best as I can tell. We need to nail it before some clown puts in a patent.
And I know very damn little on the science aspect of this, but my lungs can detect the truth..
So it seems that what is going on as we heat (flower, bubble, rosin, oil, etc) the material the compound react to the temperature and ambient pressure. The "lighter" compounds go at a lower temp and the "heavier" compounds at a higher temp. We can assume the pressure is constant in whatever the material is being heated in.
Following from here: https://chem.libretexts.org/Bookshe...nd_Intermolecular_Forces/11.5:_Vapor_Pressure
If a liquid is in an open container, however, most of the molecules that escape into the vapor phase will not collide with the surface of the liquid and return to the liquid phase. Instead, they will diffuse through the gas phase away from the container, and an equilibrium will never be established. Under these conditions, the liquid will continue to evaporate until it has “disappeared.” The speed with which this occurs depends on the vapor pressure of the liquid and the temperature. Volatile liquids have relatively high vapor pressures and tend to evaporate readily; nonvolatile liquids have low vapor pressures and evaporate more slowly.

So looking at that chart and imagining it has all of the compounds we care about (some probably lump together) we can see that individual items have vapor "smell" even below the boiling point (vapor pressure is same as local atmosphere). That is why a bag can be sniffed for "quality" - the lower boiling point items are making vapor. Leave the bag sitting and they may all go away leaving thicker vapors to make smell.
I would love to see vapor pressure charts for the desired compounds and be able to see what I get at 300, 360, 420. At 360 there is a lot left behind and that goes into edibles. 420, burned out I think. I also suspect that folks that are heating their material at a high temp to get max vapor are also reaching out to the thicker compounds.
If you look at the log pressure / temp chart on this link, and imagine vaping at 20*, you would get all the lower things in poportion to their individual vapor pressures, and miss entirely the upper two. The difference in strins is the profile of these lines I think.
https://en.wikipedia.org/wiki/Vapor_pressure
IMO this needs some discussion. There is much on the web that dances around this as best as I can tell. We need to nail it before some clown puts in a patent.
And I know very damn little on the science aspect of this, but my lungs can detect the truth..