The.Cook
Member
Growing is something strange as it can be as much complicated as you want. Beside that, some growing methods needs a better understanding of the involved chemical processes than others. Coco coir (in my opinion) is one of them.
"Coco Coir tend to adsorb Calcium and Magnesium", "pH of nutrient solution should always be adjusted to the correct value", "checking drainage ec and pH is also important" as well as "checking drainage pH and ec makes no sense as coco holds nutrients and modify pH", "Coco is an inhert media"or "Coco has high CEC" are only few exemples of how many confusing infos can be found on the internet.
If you haven't heard none of this rules before, you are likely to have never grown in coco or at least, you were lucky enough to not need search the web for hints and tips.
Some of those rules are true, others are not, but in most cases they're only simplicistic aproximations of reality.
So, are you ready?
Step 1, the basics: pH, salts and Ions (aka the good, the Bad, the Ugly)
"Daddy, why seawater tastes that baaad?"
"Ohhh it's easy my son, it's cuz there is salt in it!"
"Wow you know lot of things daddy, but what is salt?"
Bump! Panic "ehm, son, why dont you go play with your friends now? It's summer! Have fun!"
Encyclopedia, Salt: elettrically neutral chemical compound, made of 2 or more Ions with opposite electrical charge.
Lets take a look at the seasalt, almost everybody knows that its chemical formula is NaCl. In its solid form is a quiet stable compound, but what happens when we put it in water?
Correct, it dissolves, but what it means?
What actually happens is that salt divides in its Ions: Na+Cl-
If we want to be exact, there is no salt in the solution, its crystalline structure got dissolved, just positive and negative Ions. Keep those last word in mind and put them in a corner, we'll need them soon.
Everything clear till now?
Good, lets go ahed.
Water!
Water has many characteristics, almost everyone quite unique. But when the talk is about Ions, two becomes particullary important: water is an amphoteric and polar compound. Lets start from the last one.
While the term "polar" could suggest something related to cold places, it actually referres to magnetic/electric poles. Saing water is "polar" simply means that its molecule is electrically asimmetric. Everybody knows water chemical formula is H2O and if you don't, please, pick up a lighter and set your hair on fire for punishment.
So 2 Hydrogen atoms and 1 Oxygen, but maybe not everyone knows that its structure it's like that:
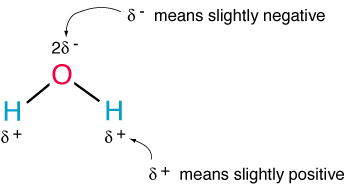
As you can see, water molecule has some sort of triangular shape, with the oxygen side been slightly (it all depends on the point of view) negative charged, and viceversa.
Could seem a little thing, but without this there would be no plants to grow.
Amphoteric is somehow a weird word. Anyway it basically means that water can act as an acid as well as a base. For definition, an Acid is something capable of releasing H+ ions, while a base is something that attract them. If put thogeter with someting more acidic, water will act as a base and viceversa
Pure water at room temperature is made of stable H2O molecules, but a really small amount "divide" to H+ and OH-
Beware that "divide" is not correct, is more of an addition:
2 H2O <--> H3O+ OH-
At the end of the day it's basically the same thing, we always have a H+ on a side, and a OH- on the other. From know on, please consider H+ and H3O+ as the same thing. In most reactions you'll see H3O+ cause it's the real form, while when talking we'll refer to H+ for easier reading.
Anyway, I sad "only a small amount". Guess how many? 1 every 10.000.000 or 1 × 10-7. Look at that "-7". Ever wondered "why pH7"? now you know.
As we have seen pH7 also mean that H+ and OH- are present in the same concentration.
If you use RO water, thats quite near what comes out from the blue hose
But if we put an acid in it, water will act as a base, bonding to an H+
H2O + HCl -> H3O+ Cl-
More H3O+ compared to OH- The pH is lowering. Ten times more H+ means 1 point less in pH.
Viceversa H2O+NH3-> OH- NH4+
More OH- compared to H+. The pH is rising.
So I've just said pure water has pH7 so same amount of H+ and OH-.
Oh, well, there's a last minute problem, like when you see there's only 10gr left and you're still in Veg: pure water is a great solvent, enough powerful to react with CO2 normally present in air to create Carbonic Acid.
H2O+CO2 -> H2CO3
Being H2CO3 an acid, water play its role as a base, actracting H+ from the newcomer and lowering pH till a 5.5 value.
Why till 5.5 and not more? I've said water act as base when toghether with someting MORE acidic. In that case, 5.5 is the equilibrium point. Maybe you are allready thinking that adding your pH+ would be a simple solution but think about it: if you raise the pH, water will be once again basic if compared to H2CO3, at that time will start to act as a base as before.
Seems frustating doesn't it? Well, actually it's a great thing: the fact is that water by its own has a very low inertia to pH adjustment. In other word, easily matches pH of surrounding substances.
Know you should start to understand why the amphoteric behavior is so important.
But what about polarity?
The answer is quite simple (or at least more simple than the amphteric thing): polarity enables water dissolve salts crystalline structure and idrate positive and negative Ions.
Look at the picture below:
Salts in a water solution are like you and your girl dancing in the middle of 1000's Bred Pit & Angelina Jolie straight in the middle of a 7th year crisys.
Few minutes later, the situation will likely be like that on the right
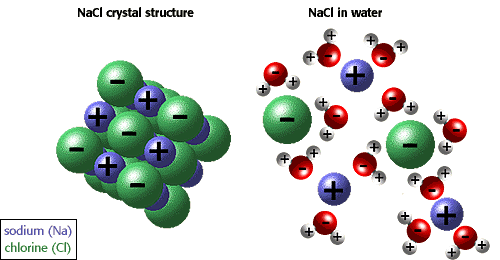
Now stop thinking angelina's green eyes for a while (yes I am a romanthic guy) and focus on female plants. Fertilizers are salts, but plants need them in their ionic form to be able to adsorb them.
Now you know what salts and Ions are, how water acts like, and what happens when those 3 are put togheter.
"Wait, but the title said pH, salts and Ions, not water, salts and ions!! What da f**k was that for??"
Well, pH is like balance between brads and angelinas. Too many Brad and only few Angelina will make them forget about the 7year crisys and try hard to reconquest the few remaining ledies. In three words: you are screwd.
If you kept reading till now, i'm quite sure you have seen this graphic before:
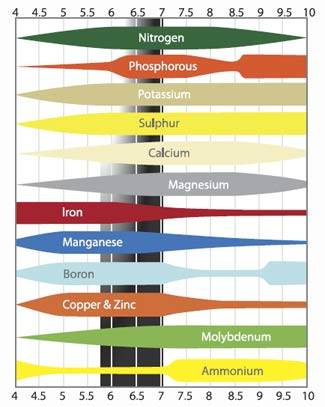
I'll not explain them one by one, but keep in mind that any significative change from pH7 could cause serius issues. As said before, 1 point more in pH means 10 times more OH- and 2 point more means 100 times more OH-, the opposite for H+ ions.
Roots use H+ and OH- to adsorb macro and micro elements, so pH balance in the nutrient solution is very important to make it possible. Beside that, different pH causes different equilibrium between ions, making possible reactions that doesn't happen under neutral conditions, resulting for example in unsolvable salts.
Undissolved salt means (quite) no ions, and no ions means no food for the plant.
"Coco Coir tend to adsorb Calcium and Magnesium", "pH of nutrient solution should always be adjusted to the correct value", "checking drainage ec and pH is also important" as well as "checking drainage pH and ec makes no sense as coco holds nutrients and modify pH", "Coco is an inhert media"or "Coco has high CEC" are only few exemples of how many confusing infos can be found on the internet.
If you haven't heard none of this rules before, you are likely to have never grown in coco or at least, you were lucky enough to not need search the web for hints and tips.
Some of those rules are true, others are not, but in most cases they're only simplicistic aproximations of reality.
So, are you ready?
Step 1, the basics: pH, salts and Ions (aka the good, the Bad, the Ugly)
"Daddy, why seawater tastes that baaad?"
"Ohhh it's easy my son, it's cuz there is salt in it!"
"Wow you know lot of things daddy, but what is salt?"
Bump! Panic "ehm, son, why dont you go play with your friends now? It's summer! Have fun!"
Encyclopedia, Salt: elettrically neutral chemical compound, made of 2 or more Ions with opposite electrical charge.
Lets take a look at the seasalt, almost everybody knows that its chemical formula is NaCl. In its solid form is a quiet stable compound, but what happens when we put it in water?
Correct, it dissolves, but what it means?
What actually happens is that salt divides in its Ions: Na+Cl-
If we want to be exact, there is no salt in the solution, its crystalline structure got dissolved, just positive and negative Ions. Keep those last word in mind and put them in a corner, we'll need them soon.
Everything clear till now?
Good, lets go ahed.
Water!
Water has many characteristics, almost everyone quite unique. But when the talk is about Ions, two becomes particullary important: water is an amphoteric and polar compound. Lets start from the last one.
While the term "polar" could suggest something related to cold places, it actually referres to magnetic/electric poles. Saing water is "polar" simply means that its molecule is electrically asimmetric. Everybody knows water chemical formula is H2O and if you don't, please, pick up a lighter and set your hair on fire for punishment.
So 2 Hydrogen atoms and 1 Oxygen, but maybe not everyone knows that its structure it's like that:
As you can see, water molecule has some sort of triangular shape, with the oxygen side been slightly (it all depends on the point of view) negative charged, and viceversa.
Could seem a little thing, but without this there would be no plants to grow.
Amphoteric is somehow a weird word. Anyway it basically means that water can act as an acid as well as a base. For definition, an Acid is something capable of releasing H+ ions, while a base is something that attract them. If put thogeter with someting more acidic, water will act as a base and viceversa
Pure water at room temperature is made of stable H2O molecules, but a really small amount "divide" to H+ and OH-
Beware that "divide" is not correct, is more of an addition:
2 H2O <--> H3O+ OH-
At the end of the day it's basically the same thing, we always have a H+ on a side, and a OH- on the other. From know on, please consider H+ and H3O+ as the same thing. In most reactions you'll see H3O+ cause it's the real form, while when talking we'll refer to H+ for easier reading.
Anyway, I sad "only a small amount". Guess how many? 1 every 10.000.000 or 1 × 10-7. Look at that "-7". Ever wondered "why pH7"? now you know.
As we have seen pH7 also mean that H+ and OH- are present in the same concentration.
If you use RO water, thats quite near what comes out from the blue hose
But if we put an acid in it, water will act as a base, bonding to an H+
H2O + HCl -> H3O+ Cl-
More H3O+ compared to OH- The pH is lowering. Ten times more H+ means 1 point less in pH.
Viceversa H2O+NH3-> OH- NH4+
More OH- compared to H+. The pH is rising.
So I've just said pure water has pH7 so same amount of H+ and OH-.
Oh, well, there's a last minute problem, like when you see there's only 10gr left and you're still in Veg: pure water is a great solvent, enough powerful to react with CO2 normally present in air to create Carbonic Acid.
H2O+CO2 -> H2CO3
Being H2CO3 an acid, water play its role as a base, actracting H+ from the newcomer and lowering pH till a 5.5 value.
Why till 5.5 and not more? I've said water act as base when toghether with someting MORE acidic. In that case, 5.5 is the equilibrium point. Maybe you are allready thinking that adding your pH+ would be a simple solution but think about it: if you raise the pH, water will be once again basic if compared to H2CO3, at that time will start to act as a base as before.
Seems frustating doesn't it? Well, actually it's a great thing: the fact is that water by its own has a very low inertia to pH adjustment. In other word, easily matches pH of surrounding substances.
Know you should start to understand why the amphoteric behavior is so important.
But what about polarity?
The answer is quite simple (or at least more simple than the amphteric thing): polarity enables water dissolve salts crystalline structure and idrate positive and negative Ions.
Look at the picture below:
Salts in a water solution are like you and your girl dancing in the middle of 1000's Bred Pit & Angelina Jolie straight in the middle of a 7th year crisys.
Few minutes later, the situation will likely be like that on the right
Now stop thinking angelina's green eyes for a while (yes I am a romanthic guy) and focus on female plants. Fertilizers are salts, but plants need them in their ionic form to be able to adsorb them.
Now you know what salts and Ions are, how water acts like, and what happens when those 3 are put togheter.
"Wait, but the title said pH, salts and Ions, not water, salts and ions!! What da f**k was that for??"
Well, pH is like balance between brads and angelinas. Too many Brad and only few Angelina will make them forget about the 7year crisys and try hard to reconquest the few remaining ledies. In three words: you are screwd.
If you kept reading till now, i'm quite sure you have seen this graphic before:
I'll not explain them one by one, but keep in mind that any significative change from pH7 could cause serius issues. As said before, 1 point more in pH means 10 times more OH- and 2 point more means 100 times more OH-, the opposite for H+ ions.
Roots use H+ and OH- to adsorb macro and micro elements, so pH balance in the nutrient solution is very important to make it possible. Beside that, different pH causes different equilibrium between ions, making possible reactions that doesn't happen under neutral conditions, resulting for example in unsolvable salts.
Undissolved salt means (quite) no ions, and no ions means no food for the plant.
Last edited:




