-
ICMag with help from Landrace Warden and The Vault is running a NEW contest in November! You can check it here. Prizes are seeds & forum premium access. Come join in!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ph question
- Thread starter eskimo
- Start date
I assume you are outdoors with a pH like that. You should be ok, using that water but if you are indoors you will need to monitor your runoff regularly. What kind of grow are you doing? 
Dime
Well-known member
I would PH adjust the water to around 7 and let it slowly change the soil PH,one of the worst things you can do is fluctuate PH as the plants can't get used to it. If it's gradual they will adapt.So I hawe this problem my soil has a pH of 8,5 and my water 5,3 do you guys think that the outcome of adding this water to the soil will be in between and so that I'm OK and in the parameters?
Thy guys, I think the way to go is to ajust a just
Anyway
Doing some platinum kush crosses from katsu
It's cheese x platinum
Gelato 45 x platinum
And (blue dream x green Crack) x platinum
Anyway
Doing some platinum kush crosses from katsu
It's cheese x platinum
Gelato 45 x platinum
And (blue dream x green Crack) x platinum
I assume you are outdoors with a pH like that. You should be ok, using that water but if you are indoors you will need to monitor your runoff regularly. What kind of grow are you doing?
Douglas.Curtis
Autistic Diplomat in Training
I strongly recommend r/o filtering any water you use with cannabis, if at all possible.
- Higher quality cannabis
- Easier pH/nutrient control
- Ability to share information with other r/o users
If you have a starting pH of 8.5 in the soil you will run into problems with iron lockout. Iron chlorosis is a yellowing of plant leaves caused by iron deficiency due to high pH. Anything over the neutral range will lock up trace minerals over the long haul. Most especially using tap water.
Below 7 pH, hydrogen is what links nutrients and makes them available. However, when the hydrogen link is finished the hydrogen disappears and the pH returns to 8.5. If you use water that is 7 pH the hydrogen will be limited and short-lived causing the nutrients to be limited. IF you use pure rainwater the pH at 5.5, which will give you enough hydrogen to release enough trace elements to grow kick-ass plants.
That's the only way I see using soil with a pH of 8.5 and avoiding Iron chlorosis.
Below 7 pH, hydrogen is what links nutrients and makes them available. However, when the hydrogen link is finished the hydrogen disappears and the pH returns to 8.5. If you use water that is 7 pH the hydrogen will be limited and short-lived causing the nutrients to be limited. IF you use pure rainwater the pH at 5.5, which will give you enough hydrogen to release enough trace elements to grow kick-ass plants.
That's the only way I see using soil with a pH of 8.5 and avoiding Iron chlorosis.
My soil, which isn’t dirt, has a Ph around 8 from the lime and oyster shells. The water is Ph to 6.3 or so. The acidity in the water helps ionize the minerals and loosen up the cations.
I think.
I think.
You are right about that. Acidity is the hydrogen that links up with nutrients. Every time there is water exposure the hydrogen will dissolve the hard elements like copper, zinc, boron, manganese, and iron which the plant can use. In a higher pH range above 7, there's no hydrogen or acidity and the trace elements become locked out. So using 5.5 pH water on an 8.5 pH substrate would be ok because the extra hydrogen in the 5.5 water would make minerals available. .My soil, which isn’t dirt, has a Ph around 8 from the lime and oyster shells. The water is Ph to 6.3 or so. The acidity in the water helps ionize the minerals and loosen up the cations.
I think.
If you look at the chart you can see how trace elements diminish as the pH rises above 7 pH. The soil in the 8s will need a lot of hydrogen or a lower pH to bring the pH down below 7 pH for a period of time. As the hydrogen dissipates the pH will rise to the original pH in the 8s.
Every time water is added there will be the release of nutrients because there's enough hydrogen and time for absorption before swinging back up.
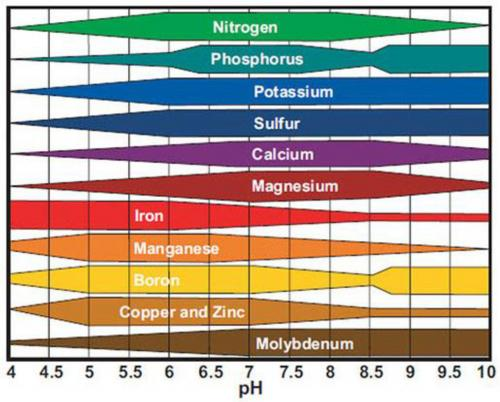
Every time water is added there will be the release of nutrients because there's enough hydrogen and time for absorption before swinging back up.
Last edited:
Yea this was also my intential thoughtIf you have a starting pH of 8.5 in the soil you will run into problems with iron lockout. Iron chlorosis is a yellowing of plant leaves caused by iron deficiency due to high pH. Anything over the neutral range will lock up trace minerals over the long haul. Most especially using tap water.
Below 7 pH, hydrogen is what links nutrients and makes them available. However, when the hydrogen link is finished the hydrogen disappears and the pH returns to 8.5. If you use water that is 7 pH the hydrogen will be limited and short-lived causing the nutrients to be limited. IF you use pure rainwater the pH at 5.5, which will give you enough hydrogen to release enough trace elements to grow kick-ass plants.
That's the only way I see using soil with a pH of 8.5 and avoiding Iron chlorosis.
St. Phatty
Active member
pH is not Linear, it is Logarithmic.
You might need to add 10 times the 6.0 pH solution to balance a given amount of 9.0 pH solution.
the 9.0 pH has 100 times the OH- of a neutral 7 solution.
The 6.0 pH has only 10 times the H+ of a neutral 7 solution.
Though that may be mis-stated; it depends on the H+ and OH- count in the neutral 7 solution.
You might need to add 10 times the 6.0 pH solution to balance a given amount of 9.0 pH solution.
the 9.0 pH has 100 times the OH- of a neutral 7 solution.
The 6.0 pH has only 10 times the H+ of a neutral 7 solution.
Though that may be mis-stated; it depends on the H+ and OH- count in the neutral 7 solution.
Without making it too complicated you can look outdoors and see what I'm talking about. Rainwater produces super growth, because of the 5.5 pH, which will allow the higher pH soil to temporarily release nutrients.pH is not Linear, it is Logarithmic.
You might need to add 10 times the 6.0 pH solution to balance a given amount of 9.0 pH solution.
the 9.0 pH has 100 times the OH- of a neutral 7 solution.
The 6.0 pH has only 10 times the H+ of a neutral 7 solution.
Though that may be mis-stated; it depends on the H+ and OH- count in the neutral 7 solution.
St. Phatty
Active member
Without making it too complicated you can look outdoors and see what I'm talking about. Rainwater produces super growth, because of the 5.5 pH, which will allow the higher pH soil to temporarily release nutrients.
Who explains it best ?
I've been listening to Prof. Kathleen Drennan's Chemistry webcasts at

MIT OpenCourseWare | Free Online Course Materials
MIT OpenCourseWare is a web based publication of virtually all MIT course content. OCW is open and available to the world and is a permanent MIT activity
ocw.mit.edu
One of her lectures touches on the theory & math of pH but it goes fast.
Would like to see her say, "OK, today I'm going to tell you how to grow Pot."
Isn’t rainwater saturated with oxygen, to the point of having peroxide? I have heard peroxide is good for gardens. I only bubble the reservoir - so far. But it gets Ph to 6.2-6.3. The hydrogen knocks stubborn ions off the cation, doesn’t it?Without making it too complicated you can look outdoors and see what I'm talking about. Rainwater produces super growth, because of the 5.5 pH, which will allow the higher pH soil to temporarily release nutrients.
Three Berries
Active member
Rain water has very little affect on pH of what it ends up usually. The more polluted it is the more it can change the pH of what is added to. Radial + charges are like steel to a magnet looking to Reduce it's charge vs Oxidation where it would add +.
So it takes very little to change the pH of rain water, either way. It's not really a good indicator.
So it takes very little to change the pH of rain water, either way. It's not really a good indicator.
Rain is just condensed water with carbonic acid in it. God makes rain and makes the best water on Earth.
Rainwater contains atmospheric gases as well as carbon dioxide, and when the carbon dioxide dissolves it forms carbonic acid, which makes the pH of normal rain about a 5.6 on the pH scale. Google
Some people don't know how to use water with a 5.5 pH and run into problems mixed with fertilizers. They either use too much calcium or not enough and have problems. They turn their noses up every time someone talks about rainwater. Using rainwater is like liquor to beer in comparison some people can handle it and some can't handle it.
Rainwater contains atmospheric gases as well as carbon dioxide, and when the carbon dioxide dissolves it forms carbonic acid, which makes the pH of normal rain about a 5.6 on the pH scale. Google
Some people don't know how to use water with a 5.5 pH and run into problems mixed with fertilizers. They either use too much calcium or not enough and have problems. They turn their noses up every time someone talks about rainwater. Using rainwater is like liquor to beer in comparison some people can handle it and some can't handle it.
Last edited:
Three Berries
Active member
I've never seen that low of pH in my rain water. It's usually 6.5 and sometimes higher. Any Nitric or Sulfur Dioxide in the air will form acids too but not much of that around anymore. Probably depends on the local pollution.
YellowCanaryConsulting
Active member
Rain is just condensed water with carbonic acid in it. God makes rain and makes the best water on Earth.
Rainwater contains atmospheric gases as well as carbon dioxide, and when the carbon dioxide dissolves it forms carbonic acid, which makes the pH of normal rain about a 5.6 on the pH scale. Google
Who explains it best ?
I've been listening to Prof. Kathleen Drennan's Chemistry webcasts at
Would like to see her say, "OK, today I'm going to tell you how to grow Pot."
Listen to enough Advancing Eco Agriculture and you can eventually unlearn most of the brain clouding, looneybin takes of the cannabis growing scene.
You can trust AEA, the only fertilizer producer who sells seed inoculant that actually works (and costs $5 an acre by their application rates.) Also the only operation selling manganese that doesn't oxidize on contact.
I'm still trying to figure out Pourbaix charts after ignoring the apparent complexity of oxidation states.
This makes sense to me:
This does not:


