-
ICMag with help from Landrace Warden and The Vault is running a NEW contest in November! You can check it here. Prizes are seeds & forum premium access. Come join in!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
~BHO LOVERS~
- Thread starter sanke
- Start date
i dont chill everything first, it makes little to no difference.
Do you use a tubes or a closed system? Soaking in warm butane(San Diego summer time) is different than blasting warm tane, contact time buddy.i dont chill everything first, it makes little to no difference.
i dont chill everything first, it makes little to no difference.
fresh frozen is a different story
just have to have an enclosed system
so sick of the tamasium neck, why such a small hole in a large collection pot is beyond my understanding.
i was wondering about fresh frozen. have never tried freezing fresh and running it yet, i guess i should. sometimes partially dried stuff gives mediocre flavor when extracted normally i find.
i dont believe butane takes on the ambient temperature of its surroundings in the can. it stays at some temperature delegated by the gasses present in the pressurized mix. of course it warms up as it exits the can, but the short time it takes to pass through the tube, regardless of local temperature it will be about the same temperature for the pass through. just try doing a run with frozen butane and material and do one at room temp with the exact same weight of the same batch of start materials maybe if you want to know for sure. i did tests involving freezing butane to -2, and -20, also with the material to be extracted, and also tried heating everything in the oven, i think we even heated butane cans
measurements showed all temperature variations to have very close to 0 effect on the final product.
Do you use a tubes or a closed system? Soaking in warm butane(San Diego summer time) is different than blasting warm tane, contact time buddy.
i dont believe butane takes on the ambient temperature of its surroundings in the can. it stays at some temperature delegated by the gasses present in the pressurized mix. of course it warms up as it exits the can, but the short time it takes to pass through the tube, regardless of local temperature it will be about the same temperature for the pass through. just try doing a run with frozen butane and material and do one at room temp with the exact same weight of the same batch of start materials maybe if you want to know for sure. i did tests involving freezing butane to -2, and -20, also with the material to be extracted, and also tried heating everything in the oven, i think we even heated butane cans
measurements showed all temperature variations to have very close to 0 effect on the final product.
I was referring to a closed system, do you have any experience with those?i was wondering about fresh frozen. have never tried freezing fresh and running it yet, i guess i should. sometimes partially dried stuff gives mediocre flavor when extracted normally i find.
i dont believe butane takes on the ambient temperature of its surroundings in the can. it stays at some temperature delegated by the gasses present in the pressurized mix. of course it warms up as it exits the can, but the short time it takes to pass through the tube, regardless of local temperature it will be about the same temperature for the pass through. just try doing a run with frozen butane and material and do one at room temp with the exact same weight of the same batch of start materials maybe if you want to know for sure. i did tests involving freezing butane to -2, and -20, also with the material to be extracted, and also tried heating everything in the oven, i think we even heated butane cans
measurements showed all temperature variations to have very close to 0 effect on the final product.
Gases have been carefully studied by physicists since the early 19th century.i dont believe butane takes on the ambient temperature of its surroundings in the can.
it stays at some temperature delegated by the gasses present in the pressurized mix.
of course it warms up as it exits the can, but the short time it takes to pass through the tube,
regardless of local temperature it will be about the same temperature for the pass through.
Thanks to their efforts all is clear and there is no room left for doubt and assumptions.
It is known that the aggregate state of gas is determined by its pressure and temperature.
If the pressure is not high enough for a given temperature, gas evaporates.
In the evaporation of gas, its volume increases, pressure increases and the temperature decreases.
When the temperature becomes low enough for the applied pressure, gas will cease to evaporate and will remain liquid.
Here is a graph of the boiling point of butane by pressure.
Gas in the can is at ambient temperature.
The graph shows that in order for the gas to remain in the liquid state at this temperature,
the pressure should be approximately the same as in an inflated car tire. And it is true.
ok i can see that. but i guess what i am referring to is the fact that the temperature decreases as the gas evaporates. as one does a tube type extraction, the tube becomes very cold very quickly, so ambient temperature would seem to have little effect.
but no sorry Hamma, i see your point about a closed system. i wasnt referring to that.
but no sorry Hamma, i see your point about a closed system. i wasnt referring to that.
Man, you got all the cool charts!Gases have been carefully studied by physicists since the early 19th century.
Thanks to their efforts all is clear and there is no room left for doubt and assumptions.
It is known that the aggregate state of gas is determined by its pressure and temperature.
If the pressure is not high enough for a given temperature, gas evaporates.
In the evaporation of gas, its volume increases, pressure increases and the temperature decreases.
When the temperature becomes low enough for the applied pressure, gas will cease to evaporate and will remain liquid.
Here is a graph of the boiling point of butane by pressure.
View Image
Gas in the can is at ambient temperature.
The graph shows that in order for the gas to remain in the liquid state at this temperature,
the pressure should be approximately the same as in an inflated car tire. And it is true.

Waterbelly
Member
So heres the story on these first three pictures, I did a mutt extraction of some Bubba, Soma A+, Cali Orange, and Harlequinn. It buddered on me in the vac. So I made an absolute out of it. It buddered on me. Did another extraction, this time some Strawberry Kush and G13. That buddered on me. Next I did an extraction of some blue widow, that buddered on me. So after having three jars of alcohol sitting in my freezer, I threw them together and vacced that. Budder. Dissolved once more, used really low temperatures, and finally got some clarity that i really wanted to show off on these first three pics. The last two are straight BW that game out looking AMAZING. I hope i did them justice, enjoy everyone.
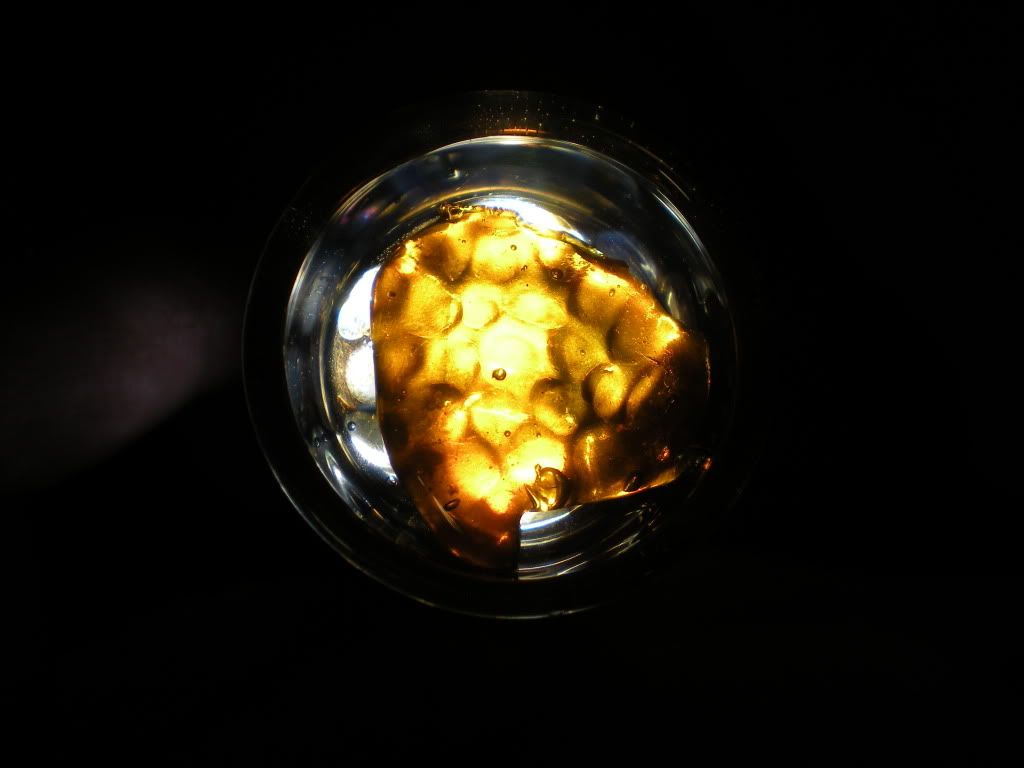









Trichgnomes
Member
The second to last one is my favorite.
The composition is just beautiful--with white backdrop, blue lipped dish and golden slab. Great job and thanks for sharing your trials and eventual success!
The composition is just beautiful--with white backdrop, blue lipped dish and golden slab. Great job and thanks for sharing your trials and eventual success!
zedd
Member
are you just acting cool with that post or what? cuz acting never cuts it..
Zedd's Vac Tek (I use a Tamisium, so there is no double boiler step for me, but 135* is fine. My tap only outputs water at about 115, so you might have to put it on the stove for a bit). I like to eliminate liquid tane before this step.
1. Preheat a glass/ceramic dish (make sure it fits in your vacuum chamber) in the oven/toaster to 150 (I like to verify temperature with an IR thermometer)
2. Cut a circle of parchment that fits inside your dish and put your oil on this. You can stick your oil in the freezer for a few seconds to solidify it and to help transfer it to the parchment. Place the parchment in the dish.
3. Lowering the viscosity of the oil completely before vaccing is important. Pretend you are melting butter in a skillet to make an omelet, and fully cover that parchment. The oil should be near-liquid.
4. I check the temperature of my oil with an IR thermometer. It usually reads around 125 at this point. I like to bring it up to about 135-140 before placing in the vac. Back in the oven/toaster for about a minute? Perhaps
5. Stick your oil in the vac, turn your pump on. Some people like to warm their pumps up beforehand because they pull harder vac.
Enjoy your terpy oil

Zedd, I purge exactly the same way!
Here is my weakness sweet tooth #3


If there was a god, he would smoke this. Taste is like pineapple grapefruit soda. Only got just over a half zip from a qp and I would do it again in a heartbeat.
Here is my weakness sweet tooth #3
If there was a god, he would smoke this. Taste is like pineapple grapefruit soda. Only got just over a half zip from a qp and I would do it again in a heartbeat.
Trichgnomes
Member
I don't dick around with purging. Think vacuum oven. It goes fast, and the oil comes out dank
To be fair, your original post that Kut was referring to specifically mentioned vacuum oven. Lots of folks do the toaster oven/vac chamber method, I have only seen photos of one person purging in a legit vacuum oven, and that is KeeferReefer on TC, and he hasn't posted in quite some time to my knowledge.
2. Cut a circle of parchment that fits inside your dish and put your oil on this. You can stick your oil in the freezer for a few seconds to solidify it and to help transfer it to the parchment. Place the parchment in the dish.
Or just get a big enough chamber (think vacuum oven
Edit: Your oil looks dank regardless. Not trying to hate, just clarify.
zedd
Member
To be fair, your original post that Kut was referring to specifically mentioned vacuum oven. Lots of folks do the toaster oven/vac chamber method, I have only seen photos of one person purging in a legit vacuum oven, and that is KeeferReefer on TC, and he hasn't posted in quite some time to my knowledge.
Or just get a big enough chamber (think vacuum oven) so you can fit your whole dish into the chamber, thus eliminating the need to scrape up underpurged oil.
Edit: Your oil looks dank regardless. Not trying to hate, just clarify.
I sometimes vac my oil directly in my tami's honeypot, which is floated in a hot water bath held at a precise temperature. I didn't post anything about this because well... most people don't have one
My dish fits in my oven. I never vac oil on the dish directly. Surface tension on glass is an issue in my experience and slows down the purge process. I always have to end up scraping and transferring to parchment for a complete purge.
Thanks homie.
These are fun! I should look for a manual online, needs a thermometer too.






