Starting older seeds can be challenging, so I've spent the last few years developing a simple and reliable method for consistently germinating them with as little fuss as possible. No more moldy seeds or fragile tangled roots on paper towels inside of ziplocks that you have to slowly and carefully open to check on - Just small, sterile, stackable, and secure containers that make it easy to monitor your progress. This should scale for any size garden.
To begin, you'll need a few things:
3.25oz portion cups with lids
Amazon

2.25" Cotton Rounds (I use Swisspers)
Amazon

A reliable temp / humidity sensor with phone notifications (I like SensorPush)
Amazon

Distilled water

Hydrogen Peroxide 3% (fresh bottle)

3ml pipette
Amazon

Directions:
Notes: You can optionally use fulvic acid and liquid seaweed extract at a rate of 0.5ml each per 100ml H20/H202 solution. For older seeds err toward 70F to slow down the pathogens. If the seeds haven't germinated at the end of 48 hours refill the portion container with fresh H20/H202 solution to rinse the seed, then remove the excess solution with a pipette and return to germination space. If you're starting multiple varieties be sure to label to the container and not the lid to avoid mix-ups. Once they have germinated use a clean spoon to transfer them from the container to your medium.
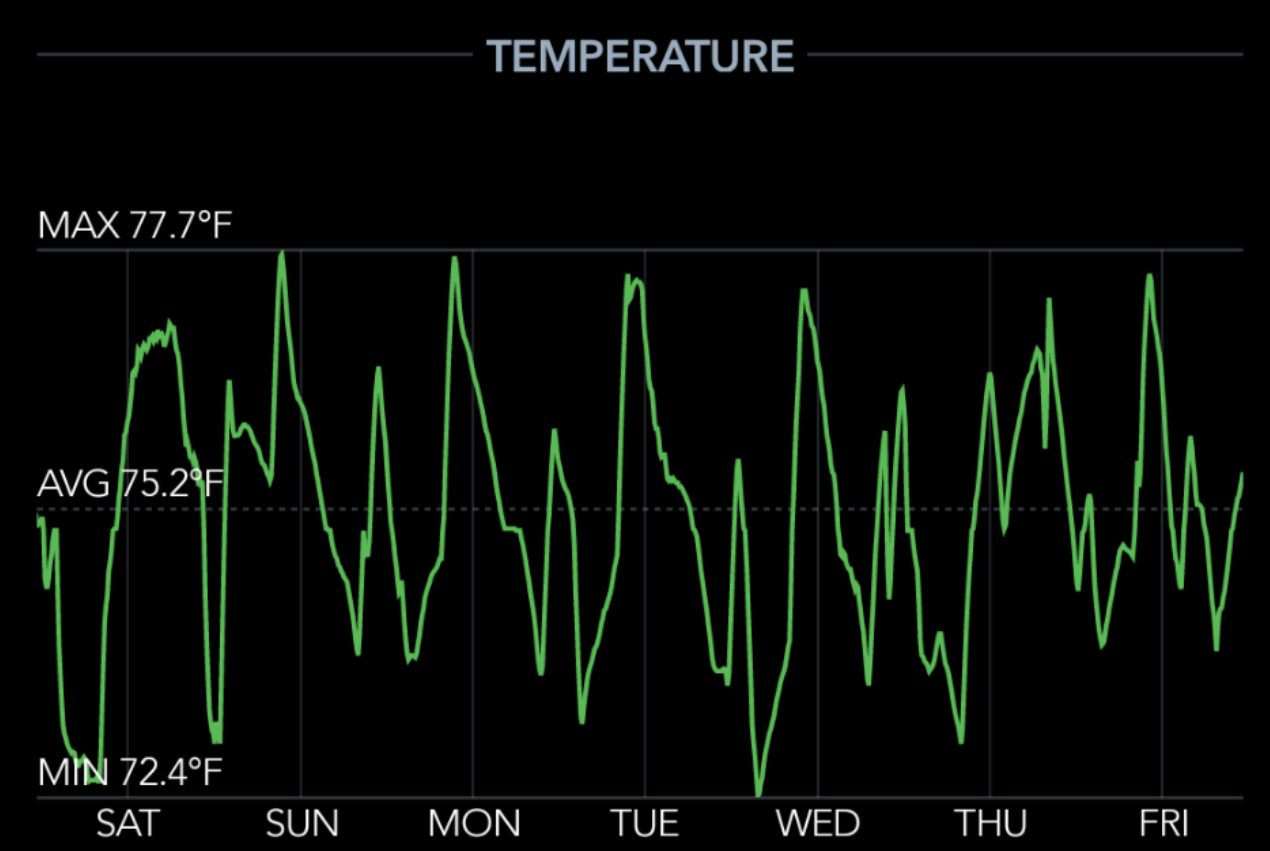
Temperature is key
Bottom's up!

After 12 hour soak

After 24hr germination

To begin, you'll need a few things:
3.25oz portion cups with lids
Amazon
2.25" Cotton Rounds (I use Swisspers)
Amazon
A reliable temp / humidity sensor with phone notifications (I like SensorPush)
Amazon
Distilled water
Hydrogen Peroxide 3% (fresh bottle)
3ml pipette
Amazon
Directions:
- Clean your hands and equipment - Sterility is key, especially with old seeds.
- Set up the temp sensor to alert you if the temperature goes below 70F or above 80F.
- Use the temp sensor to find somewhere dark that stays 70-80F consistently.
- Combine 2 parts distilled water to 1 part 3% hydrogen peroxide.
- Put 1 cotton round in the bottom of a portion cup.
- Fill the portion cup appx halfway with the H20/H202 solution you just made.
- Drop your seeds in and close the lid. Soak for 12-24hrs in the dark at 70-80f.
- Use the pipette to remove the excess solution.
- Leave the seeds on top of the moist cotton round - Do not cover them.
- Replace the lid, leave in a dark 70-80f space until they germinate (appx 24-48hrs).
Notes: You can optionally use fulvic acid and liquid seaweed extract at a rate of 0.5ml each per 100ml H20/H202 solution. For older seeds err toward 70F to slow down the pathogens. If the seeds haven't germinated at the end of 48 hours refill the portion container with fresh H20/H202 solution to rinse the seed, then remove the excess solution with a pipette and return to germination space. If you're starting multiple varieties be sure to label to the container and not the lid to avoid mix-ups. Once they have germinated use a clean spoon to transfer them from the container to your medium.
Temperature is key
Bottom's up!
After 12 hour soak
After 24hr germination
Last edited:


 thanks for suggestions on this.
thanks for suggestions on this.


