undercalisun
Member
i just got a refractometer, to understand the brix levels in my plants, i did know know where to post this thread, im trying to learn more on how to increase my brix level. it reads about 9. thank you


I used to use a pair of Vise Grips and 2 metal pates. Worked great for the couple drops that were needed.Today I got no data, because trying to extract a couple of drips from a leaf, isn't as easy as it at first sounds. Labs freeze and defrost with liquid nitrogen, to break the cells. Having first put the leaves in syringes, which are then good enough to do the crushing. Me, I'm stacking cm squares on metal plates, to crush with G-clamps. All I got was a sore hand today. I need to do better. Enough leaves and a garlic press might work, but I can spare just one a day, and never had a decent bench vice
Oh, you have had a pop at this. What range of numbers were you seeing? It's so rarely spoke about, that I don't really have an affirmed targetI used to use a pair of Vise Grips and 2 metal pates. Worked great for the couple drops that were needed.
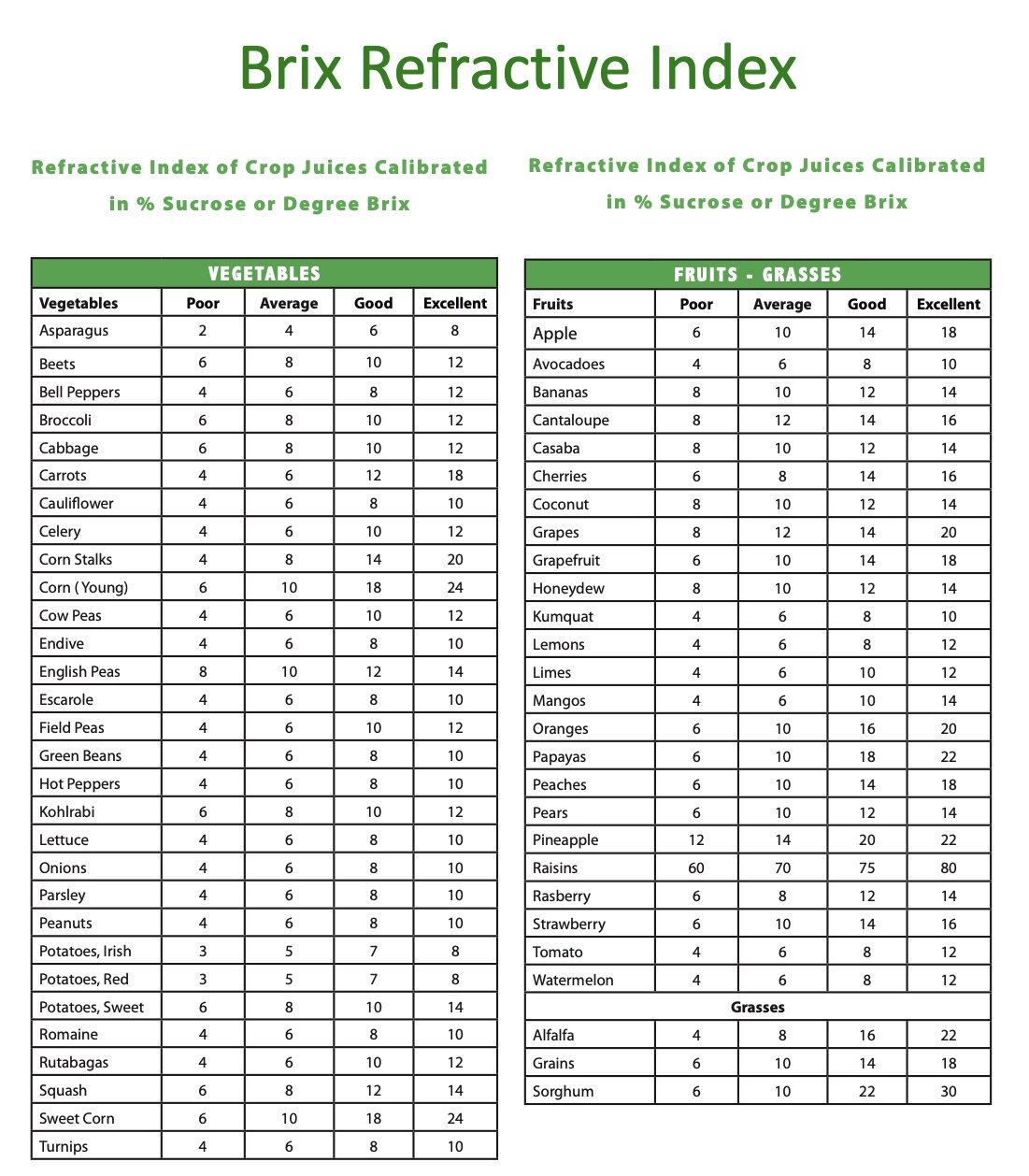

Based on brix tests on healthy cannabis plants that have produced a high quality product, a reading of 20 brix would be considered a healthy cannabis plant, but a lower brix reading does not give any direct indication as to what could be adversely affecting the plant whether its pest pressure, disease, water stress, or any other potential issues
The Mazda is retired a few years now, it was scary fun! Now it’s a BMW 5 series. More fun but waaaay more comfortable@Turbo5 Renault or Mazda?
Back to the poor plants. I let them dry back a bit, but they still looked about the norm. I did a double freeze, and timing the second freeze, made me realise how little time it takes. Just time for me to contemplate how I should of asked for her number, and snap out of it.
The squeeze was easy. Lots more extract than no freeze, and I didn't even cut it up, I just folded it. It had a different brown tint to the drops, and though the staining it left was green, it was not as bright like the good plant. I think I'm looking at a bacterial or fungal problem, but the dry-back is because I'm letting them go. I want the coco to be drier and more manageable.
I got a 13% read. A bit more than the 11% last time. Maybe because the EC is higher.
I think if all other variables remain constant, then the Brix tester serves as a plant sap EC guide. The idea it's about sugars should be foremost, but so far, I'm just keeping that in mind. I see changes related to EC
I think this is a rather crude analysis of sap. It's literally about how much the light bends, as it passes though. Like using a spear to fish, the light bends as it moves through the waters surface, so the fish appears to be somewhere else. The higher the dissolved solids, the more the fish is moved from it's place. In this instance, we see sugar as effecting the waters thickness the most, but it's not the only player.
I don't see any lengthy articles on this regarding cannabis. The general chart above, suggests 13s not bad. They are bad though, and the best article I found, suggested plants that did well, all seemed to of had 20% readings. Now that is pretty high, and would make our plant compete only with pineapples. Which is really sticky..
I have been reading your post and have gained a lot of insight, especially the last one about older plants and ec and humidity. I feel like that’s exactly what I’m seeing in especially one of my clones I have going right now.@Turbo5 Renault or Mazda?
Back to the poor plants. I let them dry back a bit, but they still looked about the norm. I did a double freeze, and timing the second freeze, made me realise how little time it takes. Just time for me to contemplate how I should of asked for her number, and snap out of it.
The squeeze was easy. Lots more extract than no freeze, and I didn't even cut it up, I just folded it. It had a different brown tint to the drops, and though the staining it left was green, it was not as bright like the good plant. I think I'm looking at a bacterial or fungal problem, but the dry-back is because I'm letting them go. I want the coco to be drier and more manageable.
I got a 13% read. A bit more than the 11% last time. Maybe because the EC is higher.
I think if all other variables remain constant, then the Brix tester serves as a plant sap EC guide. The idea it's about sugars should be foremost, but so far, I'm just keeping that in mind. I see changes related to EC
I think this is a rather crude analysis of sap. It's literally about how much the light bends, as it passes though. Like using a spear to fish, the light bends as it moves through the waters surface, so the fish appears to be somewhere else. The higher the dissolved solids, the more the fish is moved from it's place. In this instance, we see sugar as effecting the waters thickness the most, but it's not the only player.
I don't see any lengthy articles on this regarding cannabis. The general chart above, suggests 13s not bad. They are bad though, and the best article I found, suggested plants that did well, all seemed to of had 20% readings. Now that is pretty high, and would make our plant compete only with pineapples. Which is really sticky..