Hi everyone! Been a long time since I posted up a tutorial.. I hope you enjoy it.. I tried to make this as clear as possible so for you experienced people sorry if it seems a bit redundant... i wanted even the newest grower to be able to use this method.
I told some people that were having some trouble in the GG#4 forum that I would post my cloning method up that I have found to get even the hardest to clone strains a very high success rate.
This method is very simple and can even be adjusted for larger production scale.
I call it HEMPY CLONING ... i took the method used for HEMPY Buckets and applied it to cloning.. it works better then I ever expected. Most strains have a 100% success rate. This has proven by hundreds of clones from different strains. Been doing this for years now.
This produces bare root clones... can be used for soil, coco, DWC, aeroponics, etc...
Water Quality:
Preferred lighting:
Temperature Range:
PLEASE POST UP YOUR PICS AND SUCCESS! And any failures or problems you may experience.. Open to suggestions as well!
Thanks SGS!
Step 1.
Clean and disinfect a tray and dome. Use new clean cups or other containers that have been washed and disinfected (remember to rinse well!)

Step 2.
Take all cuttings,, make them as big and long as possible for you to work with... put them in cool (not to cold!) water.

Step 3.
Prepare the cup or container by cutting a hole about 1/3 of the way up from the bottom. Usually no higher than 2 in. (5 cm).
Label if needed.
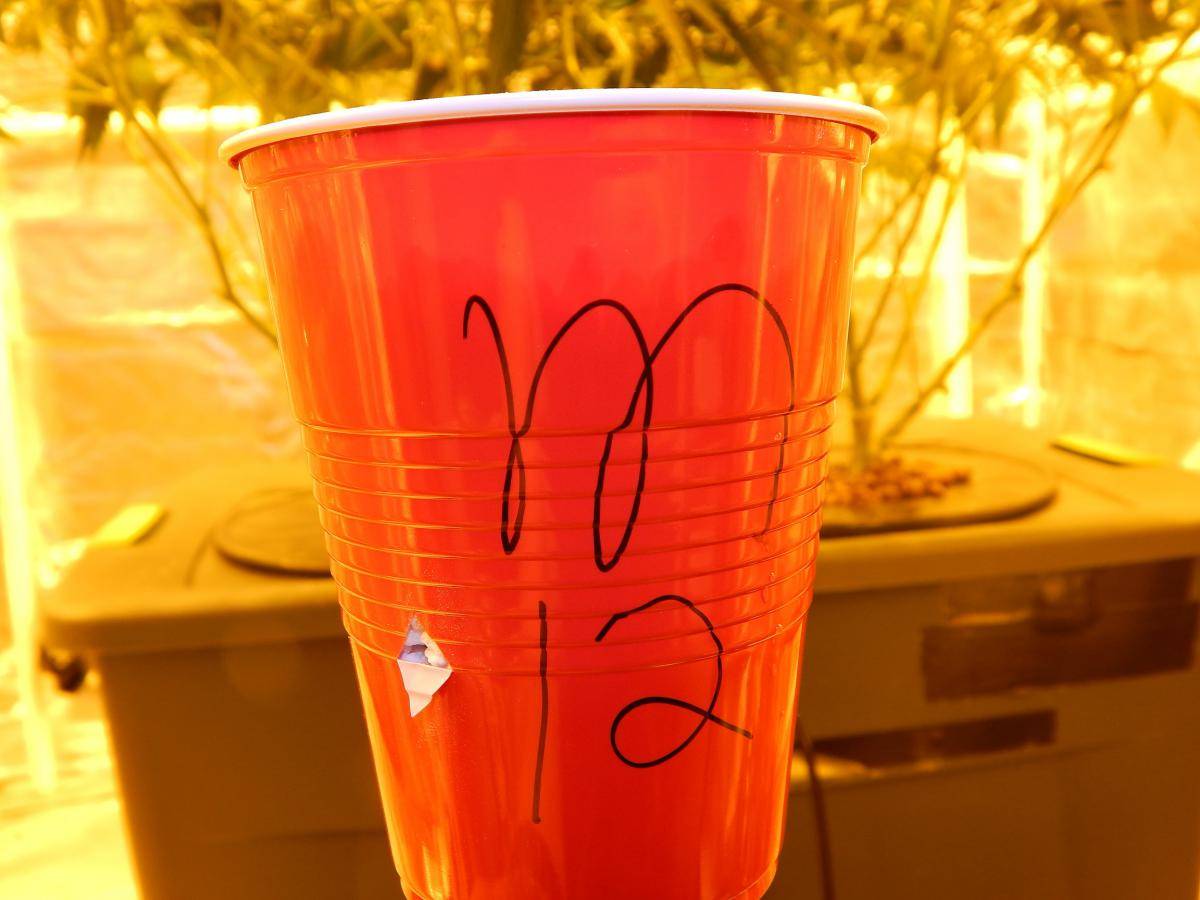
Step 4.
Fill Cups with Perlite loosely do not compact. Place in tray and water evenly to ensure all perlite gets saturated. Add until water starts to pour from hole.


I told some people that were having some trouble in the GG#4 forum that I would post my cloning method up that I have found to get even the hardest to clone strains a very high success rate.
This method is very simple and can even be adjusted for larger production scale.
I call it HEMPY CLONING ... i took the method used for HEMPY Buckets and applied it to cloning.. it works better then I ever expected. Most strains have a 100% success rate. This has proven by hundreds of clones from different strains. Been doing this for years now.
This produces bare root clones... can be used for soil, coco, DWC, aeroponics, etc...
Water Quality:
Preferred lighting:
Temperature Range:
PLEASE POST UP YOUR PICS AND SUCCESS! And any failures or problems you may experience.. Open to suggestions as well!
Thanks SGS!
Step 1.
Clean and disinfect a tray and dome. Use new clean cups or other containers that have been washed and disinfected (remember to rinse well!)
Step 2.
Take all cuttings,, make them as big and long as possible for you to work with... put them in cool (not to cold!) water.
Step 3.
Prepare the cup or container by cutting a hole about 1/3 of the way up from the bottom. Usually no higher than 2 in. (5 cm).
Label if needed.
Step 4.
Fill Cups with Perlite loosely do not compact. Place in tray and water evenly to ensure all perlite gets saturated. Add until water starts to pour from hole.
Last edited:

 Please post up your results!
Please post up your results!



