Due to the overwhelming response about the use of {H20} I have decided to make this post that was and is in my grow thread (AK-47 & HDF Side By Side Bio-Bucket Style!!!) documention it’s own thread, enjoy!!
Ok, well now let’s get this thing started!!
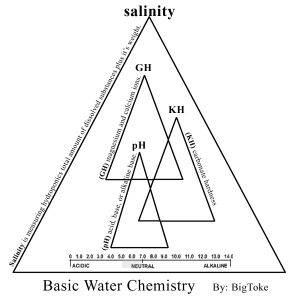
Let’s speak for a moment about what I feel that is mostly every ones problems with (pH), as I have said before every hydroponics system wither it is store bought or (DIY) must be built around two things!!
Now that you’ve determined that your water type let’s say is (Hard). Hard water forms when naturally occurring minerals enter water sources. Over time, these minerals are absorbed by groundwater. The two most common types of minerals found in hard water are calcium and magnesium compounds. The term "hard water" was originally coined to refer to water that was difficult to work with. Hard water requires much more soap, shampoo or detergent than soft water, so your soap products don't stretch nearly as far. The effects of hard water are felt most often in daily household activities such as cleaning. The minerals present in hard water inhibit soap's lathering and cleaning capabilities.
All that means is, your water has a certain amount of (calcium and magnesium) in the water, now the way you determine this is by the ppm’s, they tell you how much (GH) are in the water, btw I think most of us already know this but I will say it again, your ppm meter is mostly reading the (GH) of your hydro-systems solution, the reason for this is that calcium and magnesium are the two larger atoms in your water/solution and therefore the most conductive mineral/element of all. According to the U.S. Geological Survey, more than 85 percent of the United States geography has hard water. I have noticed that a lot of growers use water softeners. Water filtration systems come in many forms. I will discuses this later on.

The next step would be for you is to understand what that means to you as a hydroponics grower in a long term recirculating system such as the Bio-Buckets. Hard water is characterized by high levels of Bicarbonates and it makes itself known in the form of calcium and magnesium. Hard water will usually have a high pH but not necessarily, this will depend on the alkalinity of your water source, this is the reason I recommend using tap-water because it has been treated to have the most stable levels of alkalinity and by using any form of water filtration system you have there by destabilized your water alkalinity levels among other things, and thereby will not hold up under long term recalculating growing conditions, but on the other head great for drinking water but were not trying to produce good drinking water for your Bio-System, were trying to produce the best stabilized water possible and the alkalinity levels tell you what that is……..are we begging to see the light yet? Well if not, don’t worry were not done yet!!!
The obvious problem for the grower is that he will be adding quite large amounts of acid on a regular basis. If using Phosphoric acid this may lead to a build up of Phosphate in the reservoir over time. High levels of P in the solution can inhibit the uptake of other salts, Zinc for instance, and cause general nutrient imbalance.
There is one thing you must remember when dealing with indoor grow/rooms. There is no such thing as a "house plant." Plants grown in interior spaces actually come from deferent regions of the world, and must adapt to less than ideal conditions in the home or grow/room. The gardener's challenge is to know the plant's environmental needs and meet them. The interaction of environmental factors and maintenance practices contribute to the health or decline of the plant.
Now let’s discuses (Soft Water). Any water that does not contain large concentrations of the dissolved minerals calcium or magnesium. This will alter both the (pH) and the electrical conductivity.
DID YOU KNOW….. the purer the water, the lower its salinity and the greater its resistance to the flow of electricity? Salinity is related to conductivity?
DID YOU KNOW….. Soft water does not occur naturally. It must be processed.
Soft water quality all depends on how the water was softened. If the softening was accomplished by an ion-exchange method it is a better process. However, if the water was softened using sodium (SALT), you do not want to use it on your plants imo. Unfortunately, most home water softeners use sodium as the softening agent. The sodium water softener replaces the calcium and magnesium (two nutritional components needed by plants) with the sodium which is toxic to plants in large quantities produced and in most cases that I’ve seen almost all growers that use soft water use it a high levels at 100% of there water to mix in with there nutrients, it would be a better idea if you mixed it like half and half, you know like half regular water (hard water) and your filtered water (soft water) that way it you would stand a better chance of balancing it out to more your plants liking. If you use bottled water on your plants, you need to find out if the water has been softened, and if so, what method was used, sodium or ion-exchange.
Now that you have determined what type of water your going to be using to grow in, it is time to order the appropriate nutrients to go with it. Now that you have your nutrients we well discuses what relation this three elements are going to play in the roll of your (pH) problems.
Ok, well now let’s get this thing started!!
Let’s speak for a moment about what I feel that is mostly every ones problems with (pH), as I have said before every hydroponics system wither it is store bought or (DIY) must be built around two things!!
- (water efficiency) the supply, demand, and runoff thereof.
- utilization of (Dissolved Oxygen). This is, supply, exchange, and replenish.
Now that you’ve determined that your water type let’s say is (Hard). Hard water forms when naturally occurring minerals enter water sources. Over time, these minerals are absorbed by groundwater. The two most common types of minerals found in hard water are calcium and magnesium compounds. The term "hard water" was originally coined to refer to water that was difficult to work with. Hard water requires much more soap, shampoo or detergent than soft water, so your soap products don't stretch nearly as far. The effects of hard water are felt most often in daily household activities such as cleaning. The minerals present in hard water inhibit soap's lathering and cleaning capabilities.
All that means is, your water has a certain amount of (calcium and magnesium) in the water, now the way you determine this is by the ppm’s, they tell you how much (GH) are in the water, btw I think most of us already know this but I will say it again, your ppm meter is mostly reading the (GH) of your hydro-systems solution, the reason for this is that calcium and magnesium are the two larger atoms in your water/solution and therefore the most conductive mineral/element of all. According to the U.S. Geological Survey, more than 85 percent of the United States geography has hard water. I have noticed that a lot of growers use water softeners. Water filtration systems come in many forms. I will discuses this later on.

The next step would be for you is to understand what that means to you as a hydroponics grower in a long term recirculating system such as the Bio-Buckets. Hard water is characterized by high levels of Bicarbonates and it makes itself known in the form of calcium and magnesium. Hard water will usually have a high pH but not necessarily, this will depend on the alkalinity of your water source, this is the reason I recommend using tap-water because it has been treated to have the most stable levels of alkalinity and by using any form of water filtration system you have there by destabilized your water alkalinity levels among other things, and thereby will not hold up under long term recalculating growing conditions, but on the other head great for drinking water but were not trying to produce good drinking water for your Bio-System, were trying to produce the best stabilized water possible and the alkalinity levels tell you what that is……..are we begging to see the light yet? Well if not, don’t worry were not done yet!!!
The obvious problem for the grower is that he will be adding quite large amounts of acid on a regular basis. If using Phosphoric acid this may lead to a build up of Phosphate in the reservoir over time. High levels of P in the solution can inhibit the uptake of other salts, Zinc for instance, and cause general nutrient imbalance.
- The first and most obvious solution is to change-out or flush regularly. This will reduce the chances of Phosphate accumulation and ensure maintenance of a good nutrient profile. Frequency of changes-outs or flushes truly depend on the volume of water/nutrient reservoir size and number of plants. In very Hard water arias however a large amount of Phosphoric acid will be needed to correct (pH) when nutrient is first made up.
- The Best Solution by far is to use a specific formulation which is usually based on more acidic components. Hard water General Hydroponics Flora Range was formulated in response to demand from growers in various areas of the United Kingdom such as London, Thames Valley and other arias with very hard water. It was formulated to correct the (pH) of alkaline water and minimize the amounts of Phosphoric Acid that are required to maintain it at correct levels. It also takes account of the other minerals to be found in Hard water use of this product will ensure the best possible results in Hard water areas.
There is one thing you must remember when dealing with indoor grow/rooms. There is no such thing as a "house plant." Plants grown in interior spaces actually come from deferent regions of the world, and must adapt to less than ideal conditions in the home or grow/room. The gardener's challenge is to know the plant's environmental needs and meet them. The interaction of environmental factors and maintenance practices contribute to the health or decline of the plant.
Now let’s discuses (Soft Water). Any water that does not contain large concentrations of the dissolved minerals calcium or magnesium. This will alter both the (pH) and the electrical conductivity.
DID YOU KNOW….. the purer the water, the lower its salinity and the greater its resistance to the flow of electricity? Salinity is related to conductivity?
DID YOU KNOW….. Soft water does not occur naturally. It must be processed.
Soft water quality all depends on how the water was softened. If the softening was accomplished by an ion-exchange method it is a better process. However, if the water was softened using sodium (SALT), you do not want to use it on your plants imo. Unfortunately, most home water softeners use sodium as the softening agent. The sodium water softener replaces the calcium and magnesium (two nutritional components needed by plants) with the sodium which is toxic to plants in large quantities produced and in most cases that I’ve seen almost all growers that use soft water use it a high levels at 100% of there water to mix in with there nutrients, it would be a better idea if you mixed it like half and half, you know like half regular water (hard water) and your filtered water (soft water) that way it you would stand a better chance of balancing it out to more your plants liking. If you use bottled water on your plants, you need to find out if the water has been softened, and if so, what method was used, sodium or ion-exchange.
Now that you have determined what type of water your going to be using to grow in, it is time to order the appropriate nutrients to go with it. Now that you have your nutrients we well discuses what relation this three elements are going to play in the roll of your (pH) problems.