Pod Racer
Member
Time for a new theory to be challenged.
The Æromatrix explored is a radically new concept that occurred to me as an entirely new way of growing hydroponic/aeroponic-ly. The design concept is based on DWC utilizing a channel of nutrient solution (a fairly large volume) as the concept is not designed for stealth or necessarily discrete growing. It is a theory that I wish to explore and will most likely evolve into a more practical design, if it does not fail completely.
The channel approximately 2' wide by 2' deep would hold the entirety of the nutrient reserve. Within the channel located at the bottom would be a long multi armed pipe (PVC would be fine 1/2") with very small holes drilled along the 'ribs' that would inject Co2 and O2 in alternating cycles—A similar cycle to high pressure Aeroponics.
The theory I have is that the Co2 Carbon loading phase would decrease the solution ph (causing greater nutrient uptake into the plant's root structure) simultaneously releasing Carbon into the solution (aiding in uptake and construction of basic molecular building blocks as plants are 47% Carbon by dry weight).
Alternating with injections of air or O2 that would continue breaking up the Carbon released and Oxygenating the nutrient solution thereby re-stabilizing it again before the next Carbon loading phase takes place.
I have no idea if or how this will work, however it is my intention to experiment.
This is 90 degrees from Aeroponic and I'm not sure what it would be classified as so I have named it Æromatrix and I intend to utilize the exact same design mirrored under the canopy with Co2 and O2 (fresh air) cycling (which should, I believe, prevent the plant from reducing its stomata coverage in response to elevated Co2 levels.)
As it is apparent that the root system in True Aeroponic Environments almost respires on a 5-minute cycle, in a submerged nutrient solution with heavy aeration, agitation, and Carbon availability the rates of growth and health should rival that of an ideal Aeroponic environment.
The nutrient strategies I have not yet gotten too, first I'm working on how this rig might be constructed and tested.
Anyone interested in running experiments of this nature would be welcomed to join in and investigate to see if we haven't actually found a uniquely different method of aeroponic respiration via carbonic action and carbon loading. How much fun will that be, eh?
The Æromatrix explored is a radically new concept that occurred to me as an entirely new way of growing hydroponic/aeroponic-ly. The design concept is based on DWC utilizing a channel of nutrient solution (a fairly large volume) as the concept is not designed for stealth or necessarily discrete growing. It is a theory that I wish to explore and will most likely evolve into a more practical design, if it does not fail completely.
The channel approximately 2' wide by 2' deep would hold the entirety of the nutrient reserve. Within the channel located at the bottom would be a long multi armed pipe (PVC would be fine 1/2") with very small holes drilled along the 'ribs' that would inject Co2 and O2 in alternating cycles—A similar cycle to high pressure Aeroponics.
The theory I have is that the Co2 Carbon loading phase would decrease the solution ph (causing greater nutrient uptake into the plant's root structure) simultaneously releasing Carbon into the solution (aiding in uptake and construction of basic molecular building blocks as plants are 47% Carbon by dry weight).
Alternating with injections of air or O2 that would continue breaking up the Carbon released and Oxygenating the nutrient solution thereby re-stabilizing it again before the next Carbon loading phase takes place.
I have no idea if or how this will work, however it is my intention to experiment.
This is 90 degrees from Aeroponic and I'm not sure what it would be classified as so I have named it Æromatrix and I intend to utilize the exact same design mirrored under the canopy with Co2 and O2 (fresh air) cycling (which should, I believe, prevent the plant from reducing its stomata coverage in response to elevated Co2 levels.)
As it is apparent that the root system in True Aeroponic Environments almost respires on a 5-minute cycle, in a submerged nutrient solution with heavy aeration, agitation, and Carbon availability the rates of growth and health should rival that of an ideal Aeroponic environment.
The nutrient strategies I have not yet gotten too, first I'm working on how this rig might be constructed and tested.
Anyone interested in running experiments of this nature would be welcomed to join in and investigate to see if we haven't actually found a uniquely different method of aeroponic respiration via carbonic action and carbon loading. How much fun will that be, eh?

Last edited:





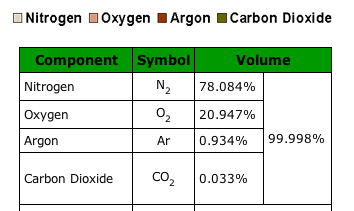
 Most these systems have the O2 have the H2O but only limited Co2 absobtion, which in water would be more saturated and available I would think. So, cycle respiriation like aeroponic, but using Co2 and O2 in place of pure O2 in mechanical formed microdroplets. Let her boil in free exchange of pure elements.
Most these systems have the O2 have the H2O but only limited Co2 absobtion, which in water would be more saturated and available I would think. So, cycle respiriation like aeroponic, but using Co2 and O2 in place of pure O2 in mechanical formed microdroplets. Let her boil in free exchange of pure elements.