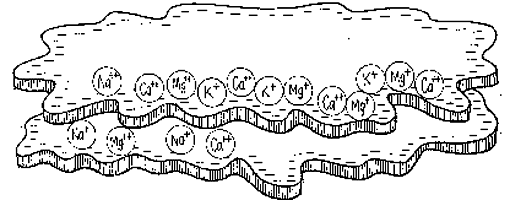
It would be nice to rename this thread soil PH changes with plant activity.
I am floundering my way through my first run. I am using 1/3 coots mix amended with BAS stuff. The plants are in 7 gallon bags and I Ph adjusted the soil to 6.8 before the start with some oyster meal. The plants appear to be doing great finally by the growth and leafs. I am using a SWICK (bags on a SIP bed), and adjusting my tap water down to 6.5 in the reservoir, and have to do so between feedings because the water exchange with the plants raises the PH of the reservoir. Like up to 6.8 or 7.0 if I let it go two cycles (12 hours).
So the soil is raising the water Ph somehow. I have a Ph/moisture meter that started working pretty well, until I inoculated the plants in bags. and then it was acting funny measuring the soil in the bags, like it is short circuiting when I test the soil in them. I wrote it off as just being more chinese junk, but today I tried it again and it seems to be working OK. Readings of my lawn, extra soil, garden... all the same as they were when I got the probe, 6.6 to 6.9.
This is what the bags the plants I'm about to flip in a week or so show...
 Yeah, that says 4.5!
Yeah, that says 4.5!
The needle goes straight to that reading like it means it. All 4 plants read about the same...
I have to believe something is going on, and I wonder if the soil has good cation exchange capacity now and is freaking out the meter.
Anybody have a clue? Here's one of the plants, growing like a weed.

I feel like I shouldn't worry and do anything drastic. How can soil that is making the water higher in Ph, read lower... way lower, than it was? The only thing I can figure is there is a lot of ion exchange going on finally in the soil in the big tent. This may be a way to know if the soil system is working properly.
I am floundering my way through my first run. I am using 1/3 coots mix amended with BAS stuff. The plants are in 7 gallon bags and I Ph adjusted the soil to 6.8 before the start with some oyster meal. The plants appear to be doing great finally by the growth and leafs. I am using a SWICK (bags on a SIP bed), and adjusting my tap water down to 6.5 in the reservoir, and have to do so between feedings because the water exchange with the plants raises the PH of the reservoir. Like up to 6.8 or 7.0 if I let it go two cycles (12 hours).
So the soil is raising the water Ph somehow. I have a Ph/moisture meter that started working pretty well, until I inoculated the plants in bags. and then it was acting funny measuring the soil in the bags, like it is short circuiting when I test the soil in them. I wrote it off as just being more chinese junk, but today I tried it again and it seems to be working OK. Readings of my lawn, extra soil, garden... all the same as they were when I got the probe, 6.6 to 6.9.
This is what the bags the plants I'm about to flip in a week or so show...
The needle goes straight to that reading like it means it. All 4 plants read about the same...
I have to believe something is going on, and I wonder if the soil has good cation exchange capacity now and is freaking out the meter.
Anybody have a clue? Here's one of the plants, growing like a weed.
I feel like I shouldn't worry and do anything drastic. How can soil that is making the water higher in Ph, read lower... way lower, than it was? The only thing I can figure is there is a lot of ion exchange going on finally in the soil in the big tent. This may be a way to know if the soil system is working properly.
Last edited: